Chitosan-bi(aryl-carbamate)-(amide) and preparation method thereof
A technology of aromatic carbamate and chitosan, which is applied in the field of chitosan-di- and its preparation, can solve the problem that the stationary phase is not long in service life, and the organic solvent cannot be used as a flow equalization, and is beneficial to hand Character recognition, strong chiral separation performance, and structural regularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Preparation of chitosan:
[0061] Grind the purchased flake chitin (shrimp shell) with a pulverizer, sieve (355 μm), collect the chitin under the sieve as raw material, and use two methods to deacetylate chitin (document 14-16); and use Chitosan can be degraded by hydrogen peroxide (References 17 and 18), and chitosan with different molecular weights can be obtained by adjusting the concentration of hydrogen peroxide and the reaction temperature. After degradation, the degree of deacetylation of chitosan remained basically unchanged.
[0062] Preparation of chitosan with n-pentanol as solvent:
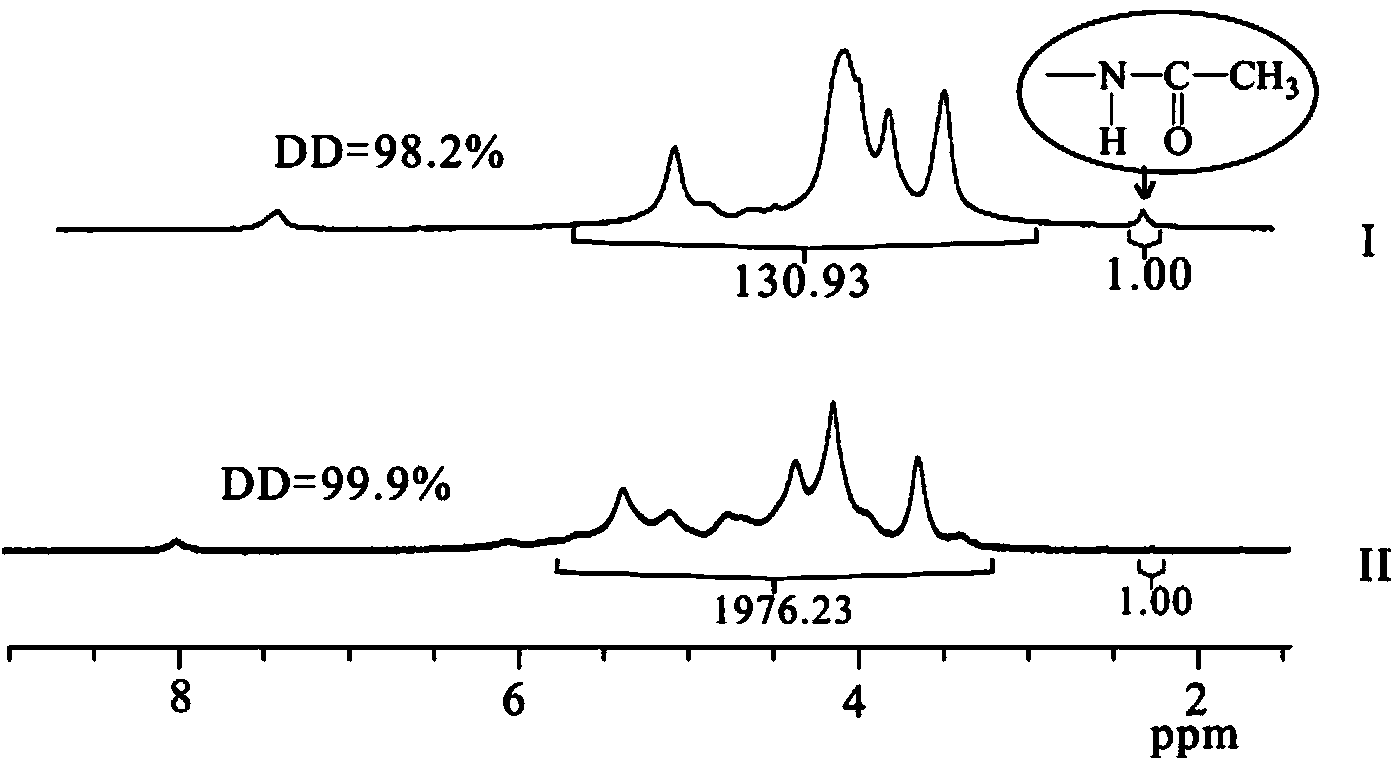
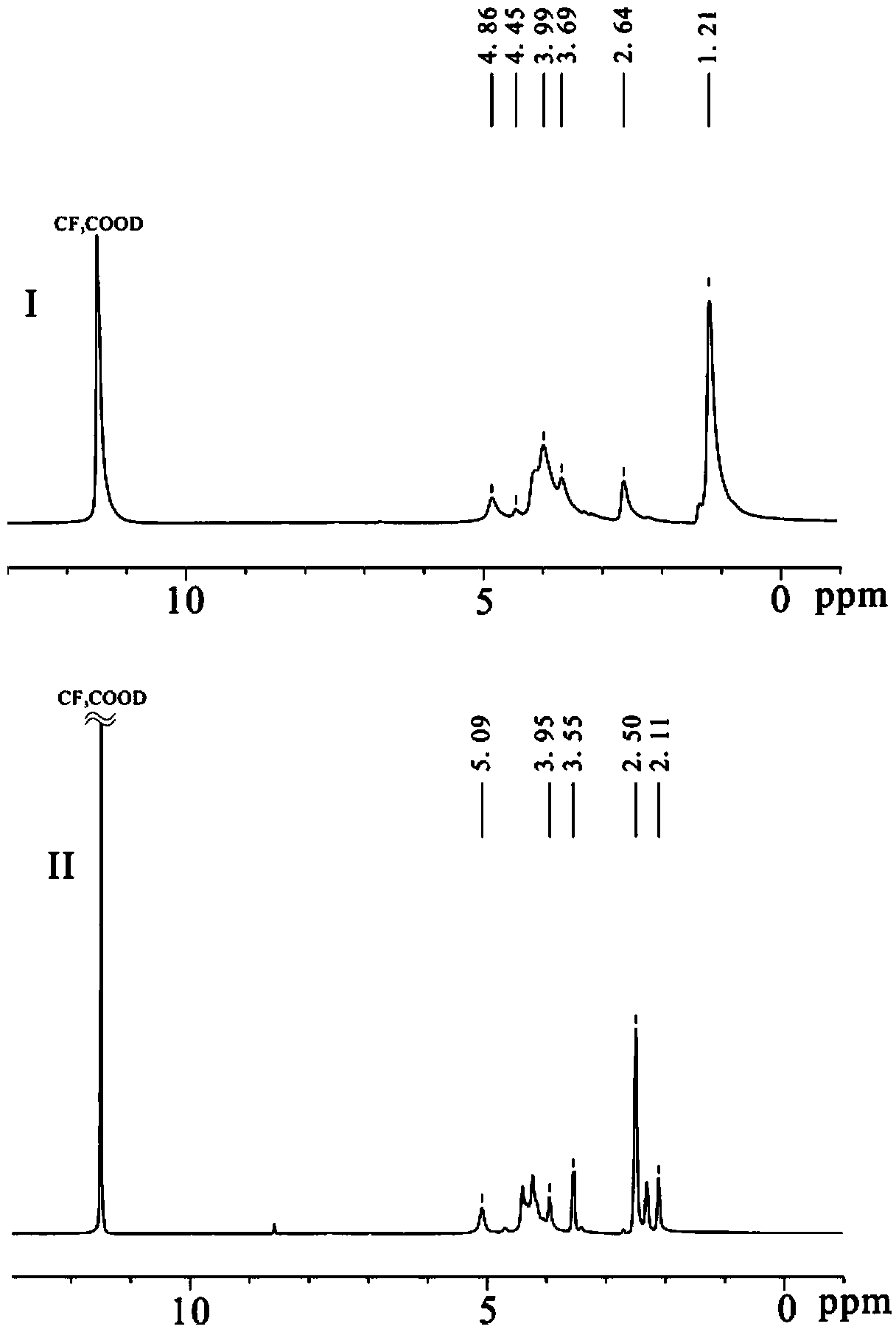
[0063] Weigh 20g of dried chitin into a three-necked flask, add NaOH and n-pentanol according to chitosan / NaOH / n-pentanol=1 / 5 / 11 (mass ratio), heat to reflux for 3h, filter, and wash with water until medium properties, drying to obtain 13.64g white solid, yield 86%; its 1 H NMR diagram is attached figure 1 As shown in (I), 1 H NMR (CF 3 COOD, δ / ppm): 5.78-3.24 (H on the gl...
Embodiment 1
[0070] Synthesis of Chitosan-bis(3-methylphenylcarbamate)-(isobutyramide)
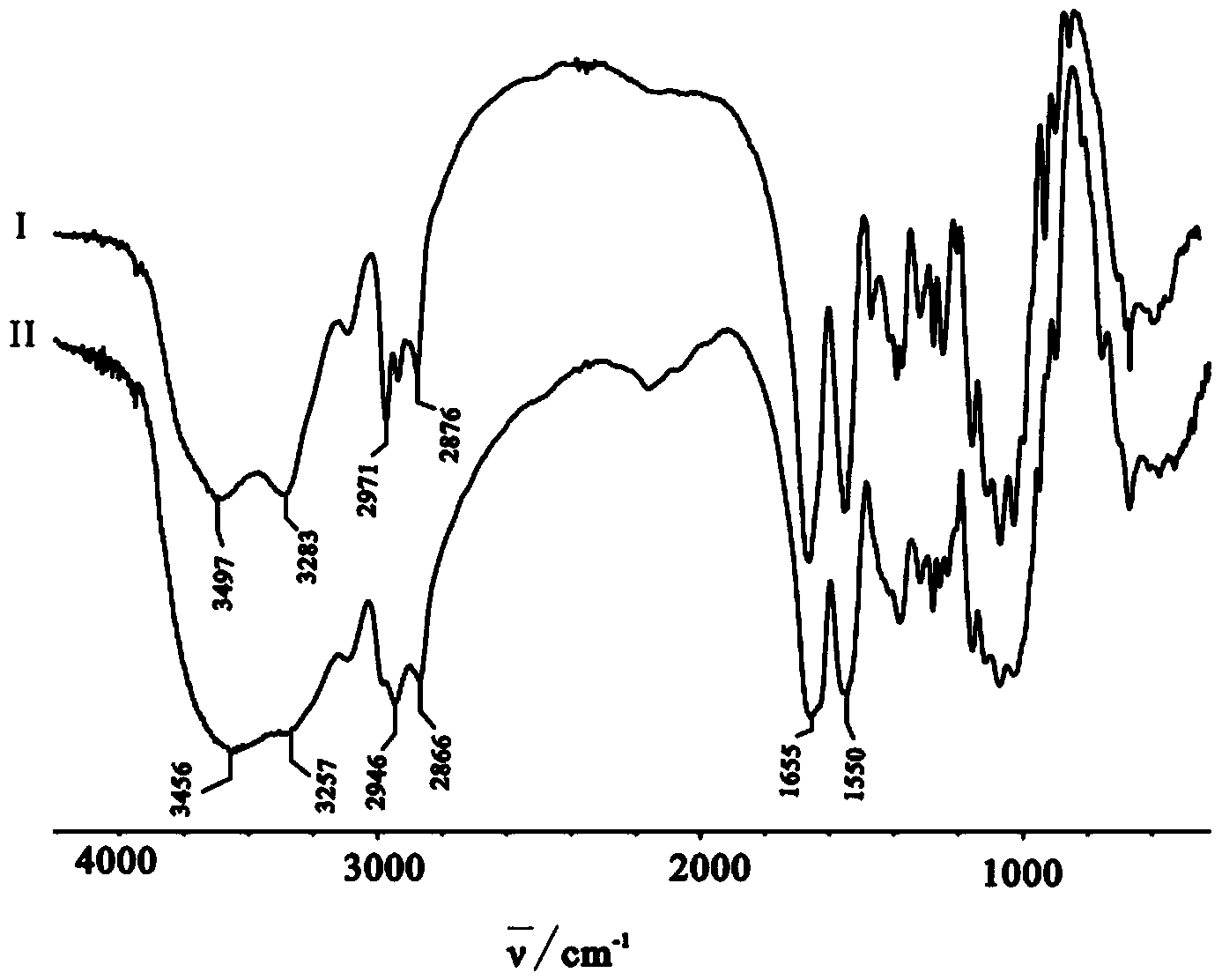
[0071] Preparation of N-isobutyrylated chitosan: 1.50g (9.32mol repeating unit) chitosan (number average molecular weight 60,000, deacetylation degree 98.2%) was added in a 250mL three-necked flask, and 0.75g isobutyric acid was added Butyric acid and 30g distilled water, stir to make chitosan dissolve until clear and transparent. Then add 70mL of methanol, after mechanical stirring, quickly add 8.84g (55.92mmol) of isobutyric anhydride (the molar ratio of anhydride to chitosan repeating unit is 6:1) and 80mL of methanol, and react at 16°C for 7h. After the reaction is complete, pour the reaction solution into 240mL 0.5mol / L KOH-ethanol solution, stir for two hours, let stand overnight, filter, wash the product with ethanol and water until neutral, and dry to obtain 2.01g of N-isobutyl Acylated chitosan, yield: 93%; infrared spectrogram as attached figure 2 (I) shown: IR (KBr, cm -1 )υ: 3523-3254(-...
Embodiment 2
[0075] Synthesis of Chitosan-bis(3-chloro-4-methylphenylcarbamate)-(cyclobutylformamide)
[0076] The preparation of N-cyclobutylformyl chitosan: 1.0g (6.21mmol repeat unit) chitosan (number average molecular weight 32,000, deacetylation degree 98.0%) is added in the 250mL three-necked flask, adds 0.60g cyclobutyl formic acid, 20g of distilled water, stirred to dissolve the chitosan until clear and transparent. Add 30mL of methanol, stir mechanically, then quickly add 7.92g (43.47mmol) cyclobutyl formic anhydride (the molar ratio of anhydride to chitosan repeating unit is 7:1) and 70mL of methanol, and react at 25°C for 7h. After the reaction was completed, the reaction solution was poured into 185 mL of 0.5 mol / L KOH-ethanol solution, stirred for two hours, and left to stand overnight. Filter, wash the product to neutrality with ethanol and water, dry, obtain 1.37g N-cyclobutylated chitosan, productive rate: 91%; Its infrared spectrogram is as attached figure 2 Shown in (I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com