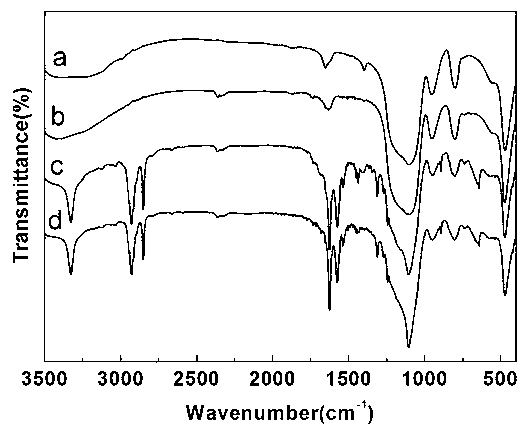
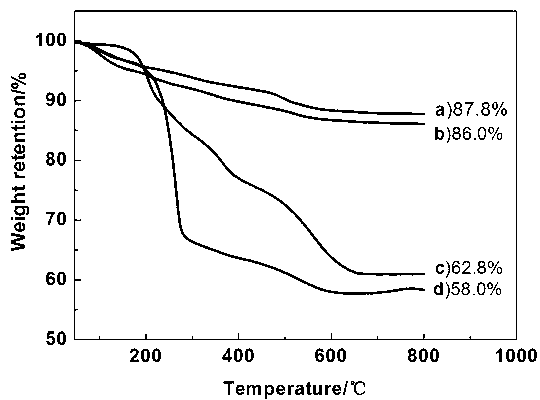
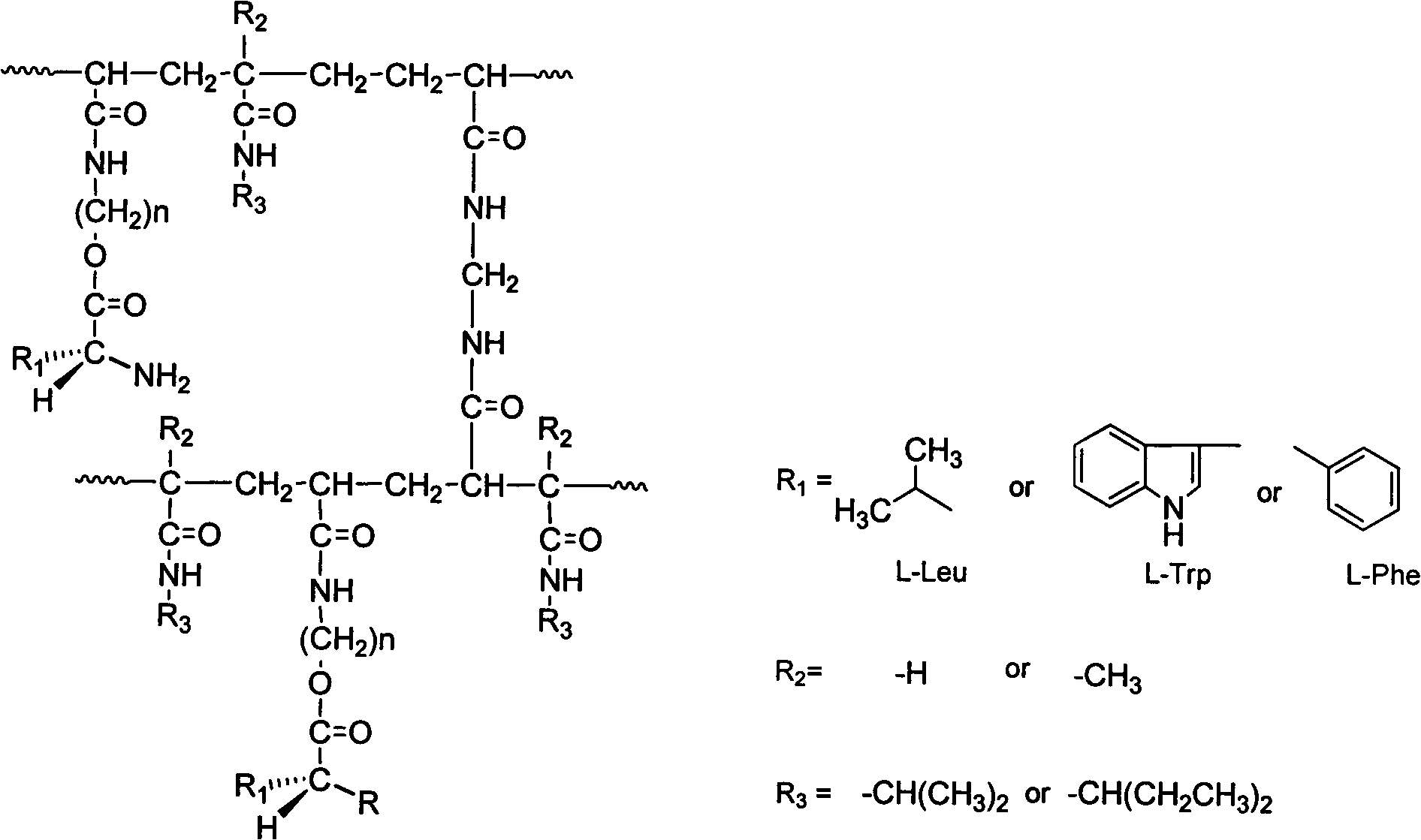
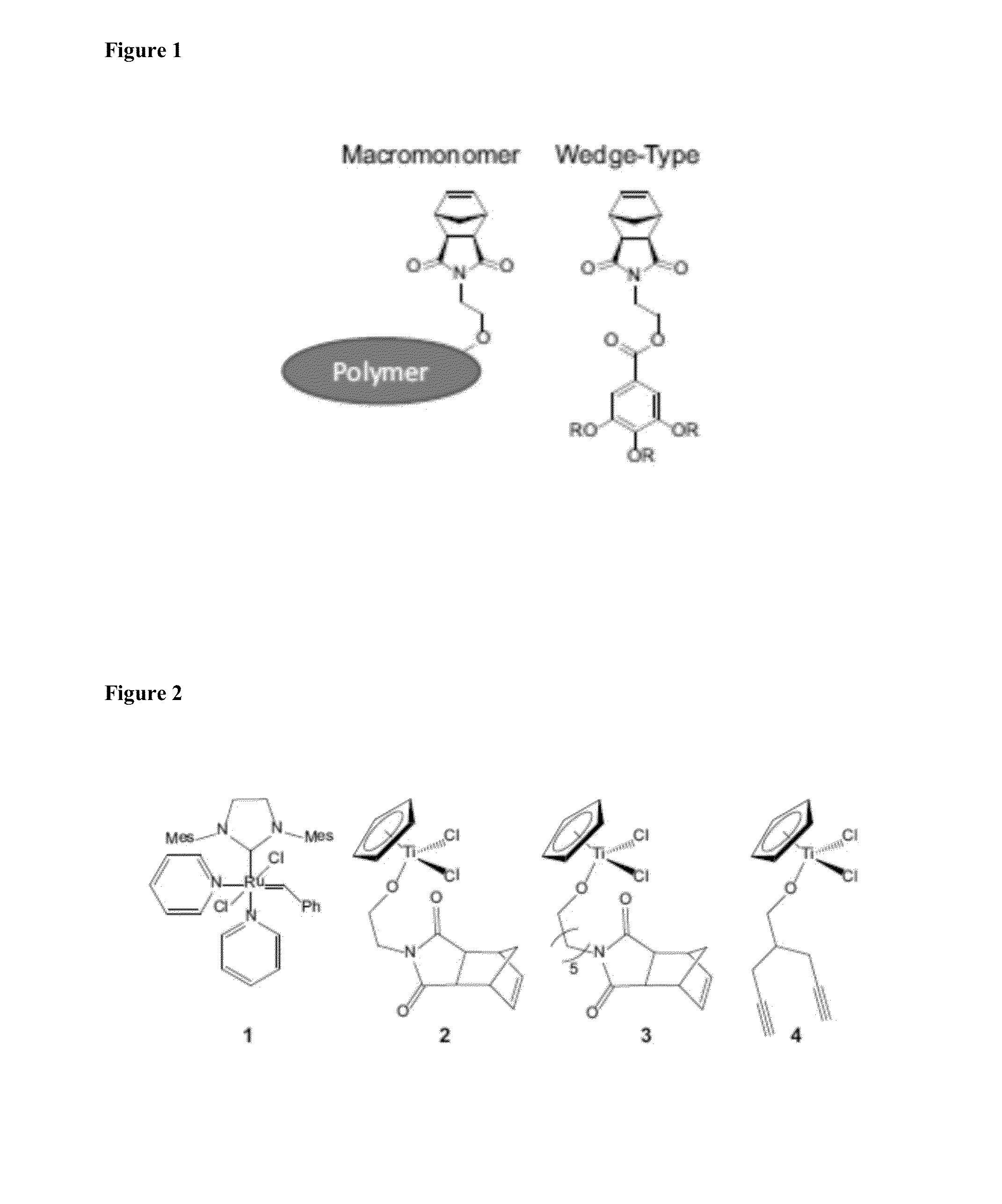
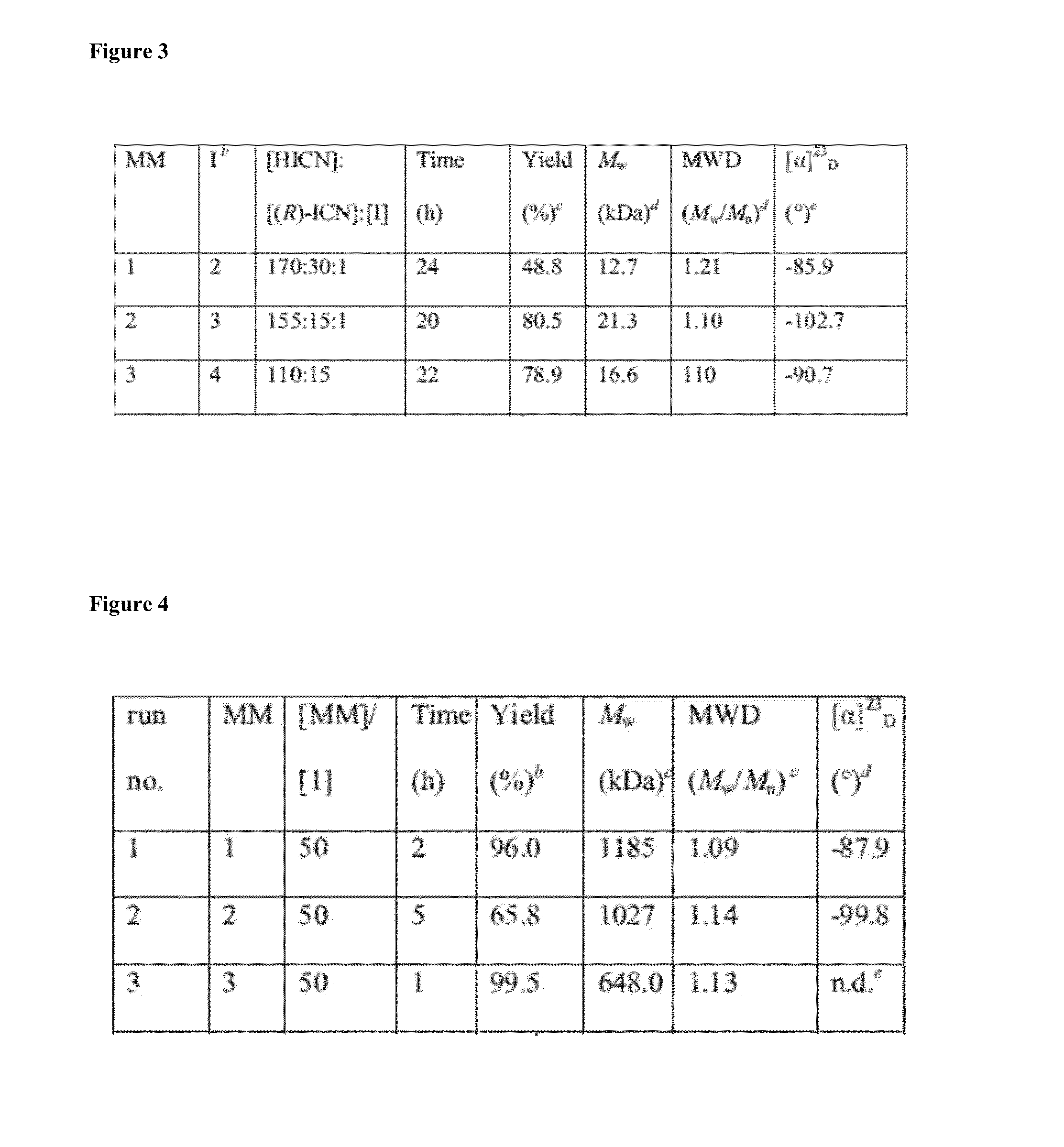
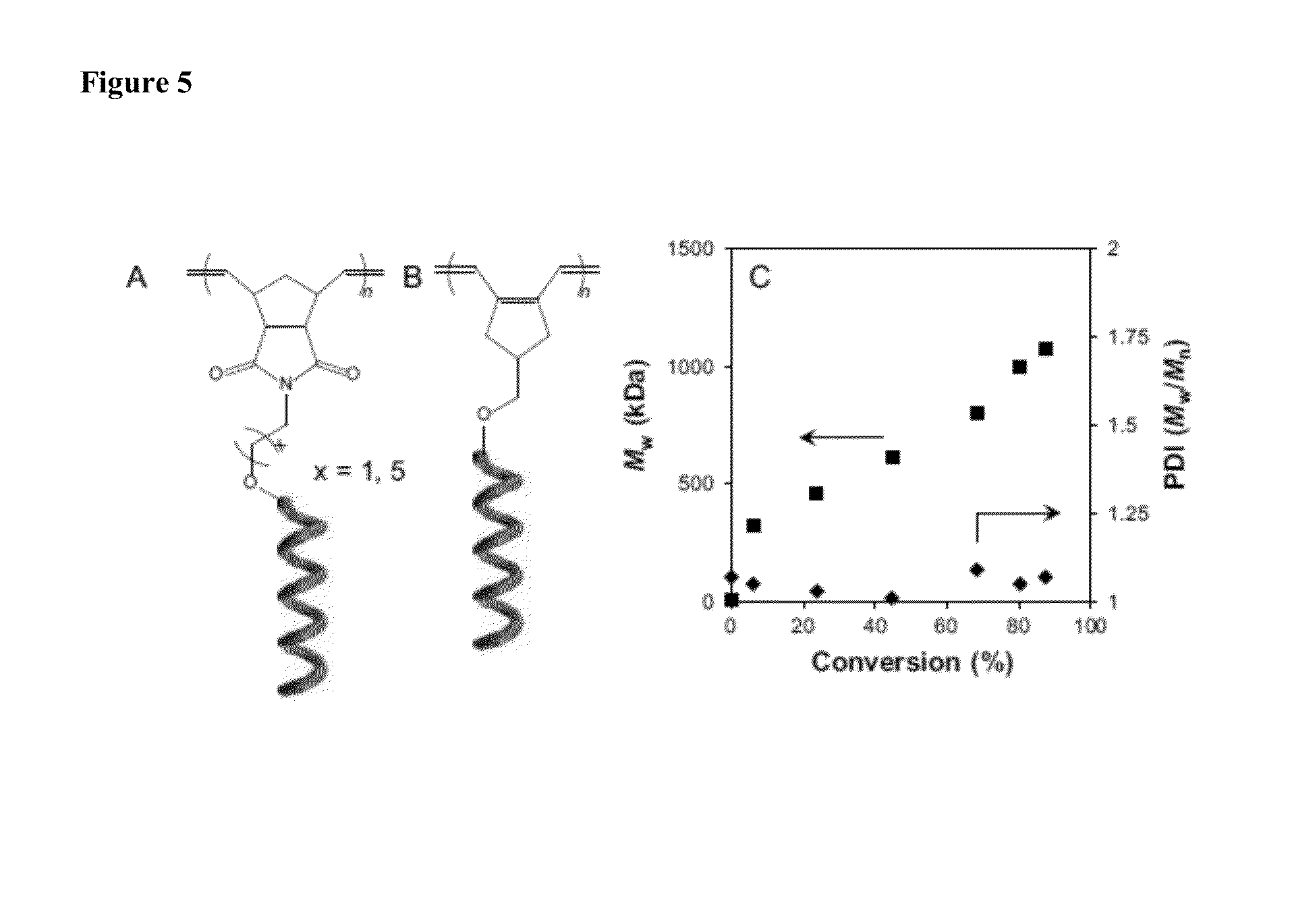
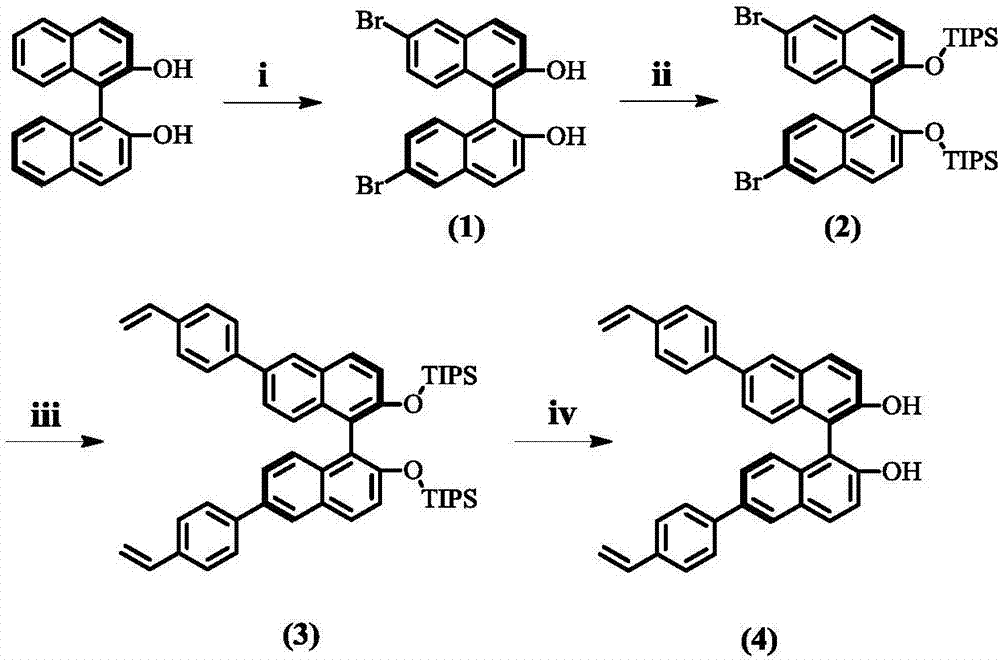
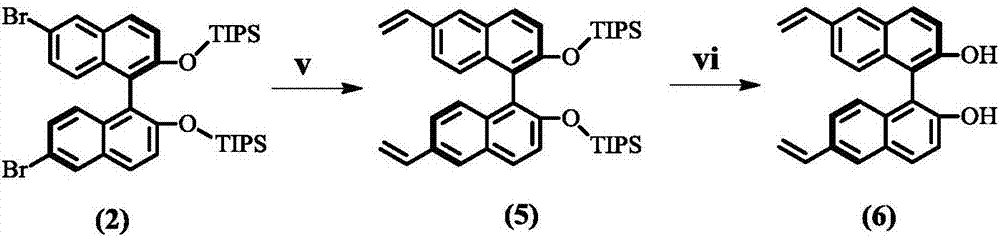
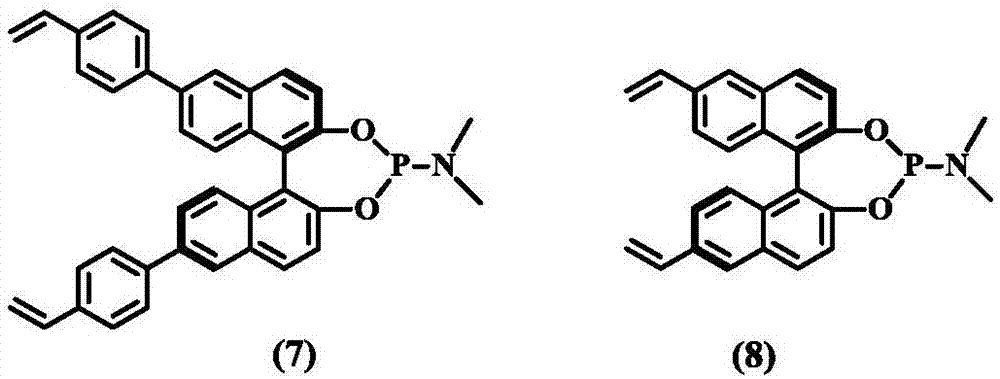
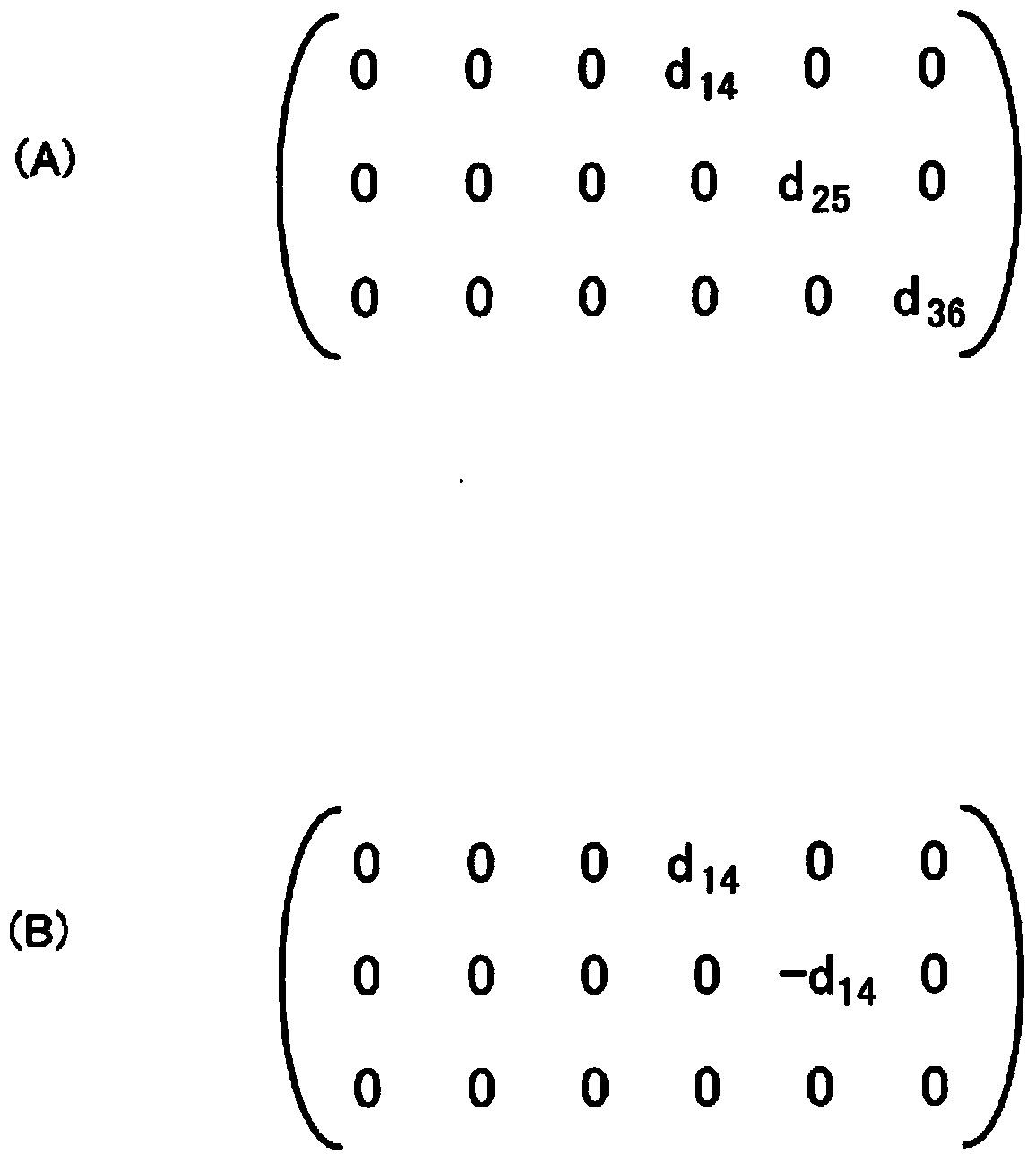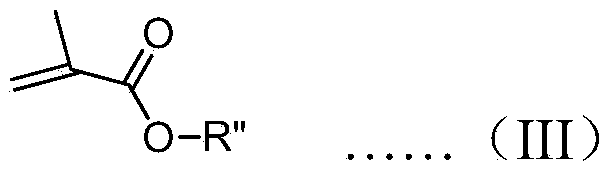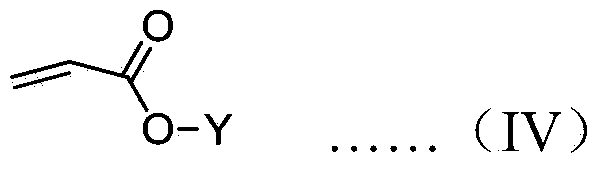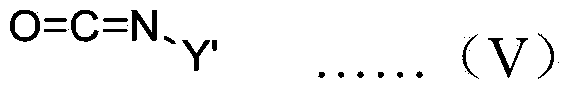Patents
Literature
80 results about "Chiral polymers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
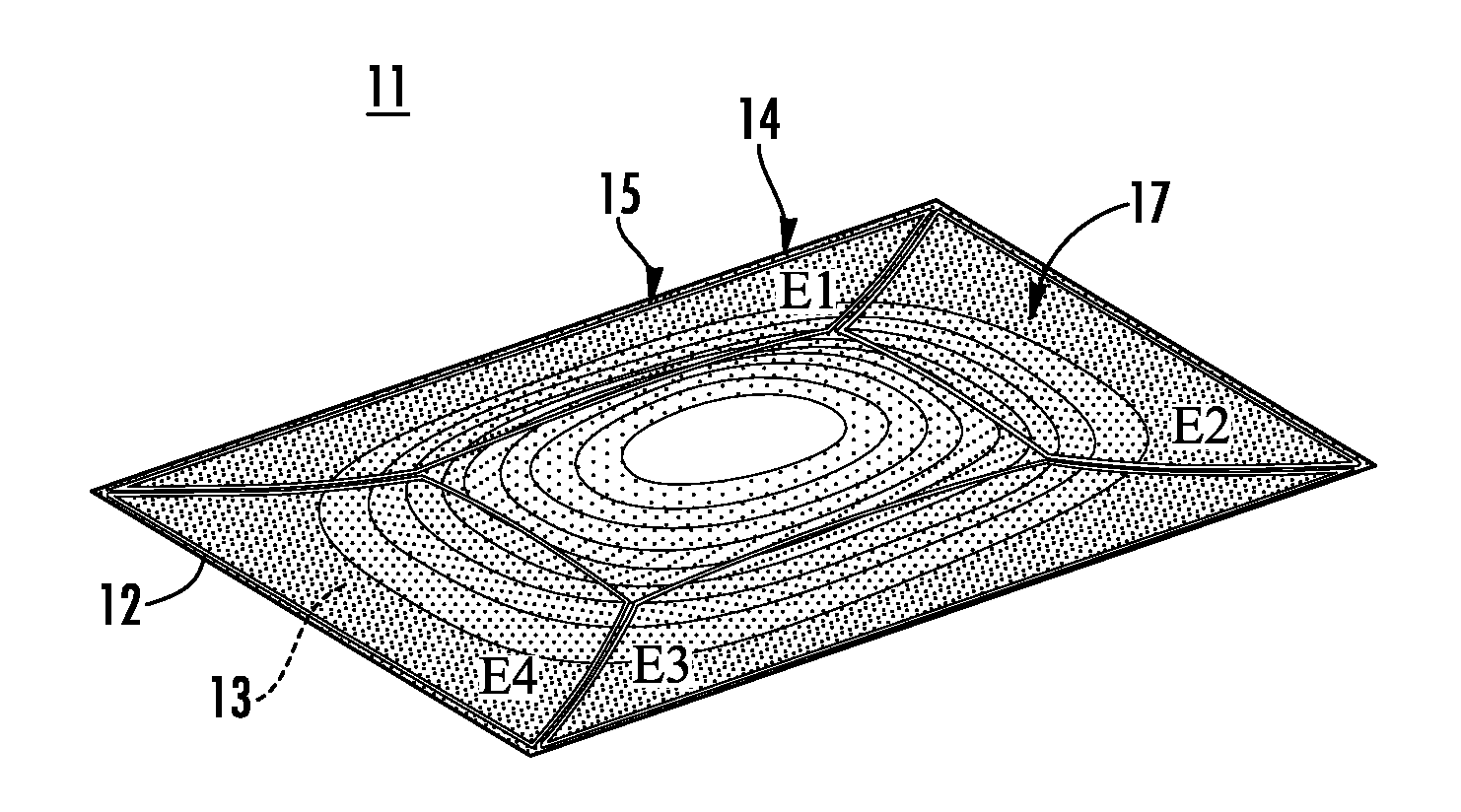
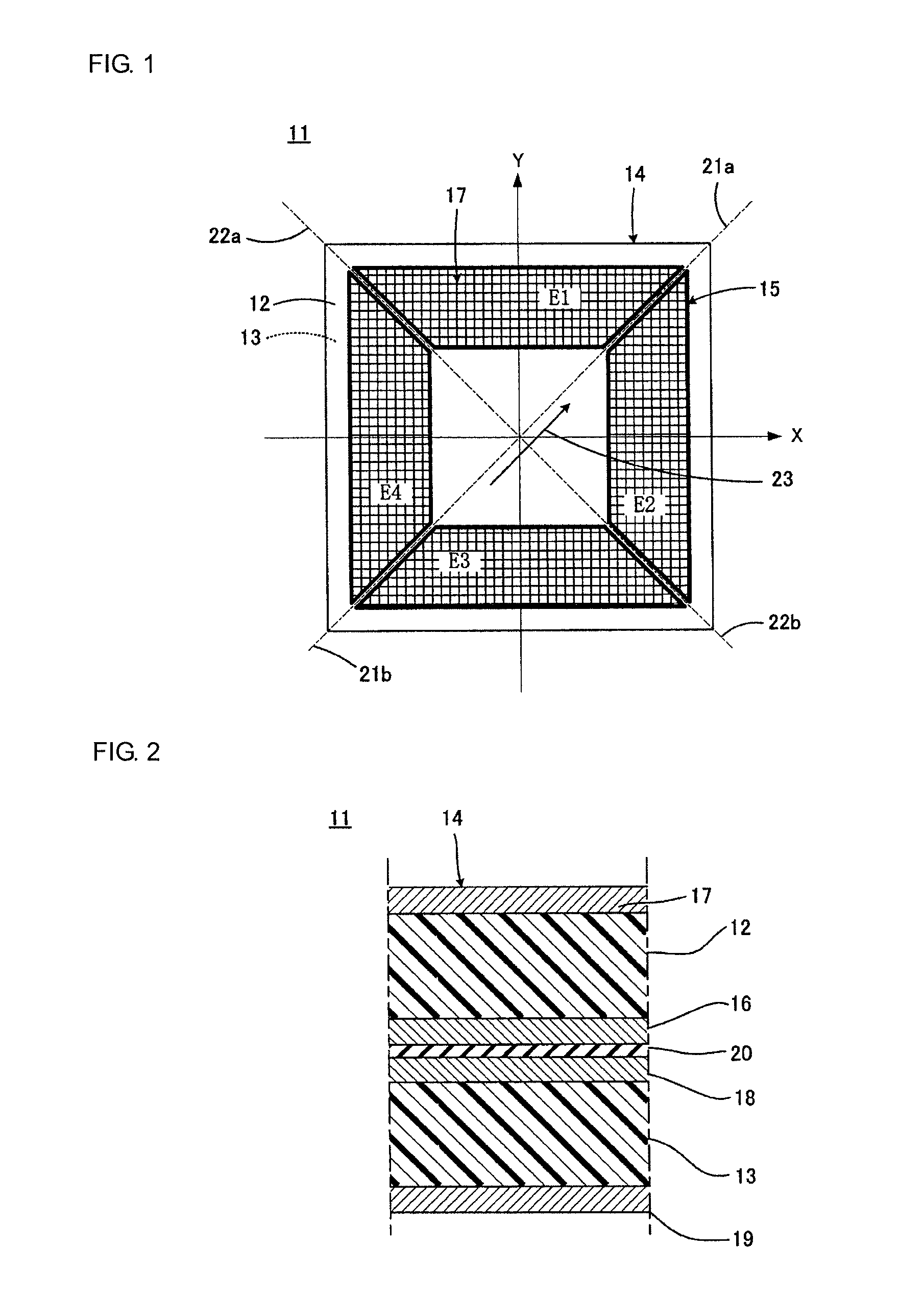
Piezoelectric polymer material, process for producing same, and piezoelectric element
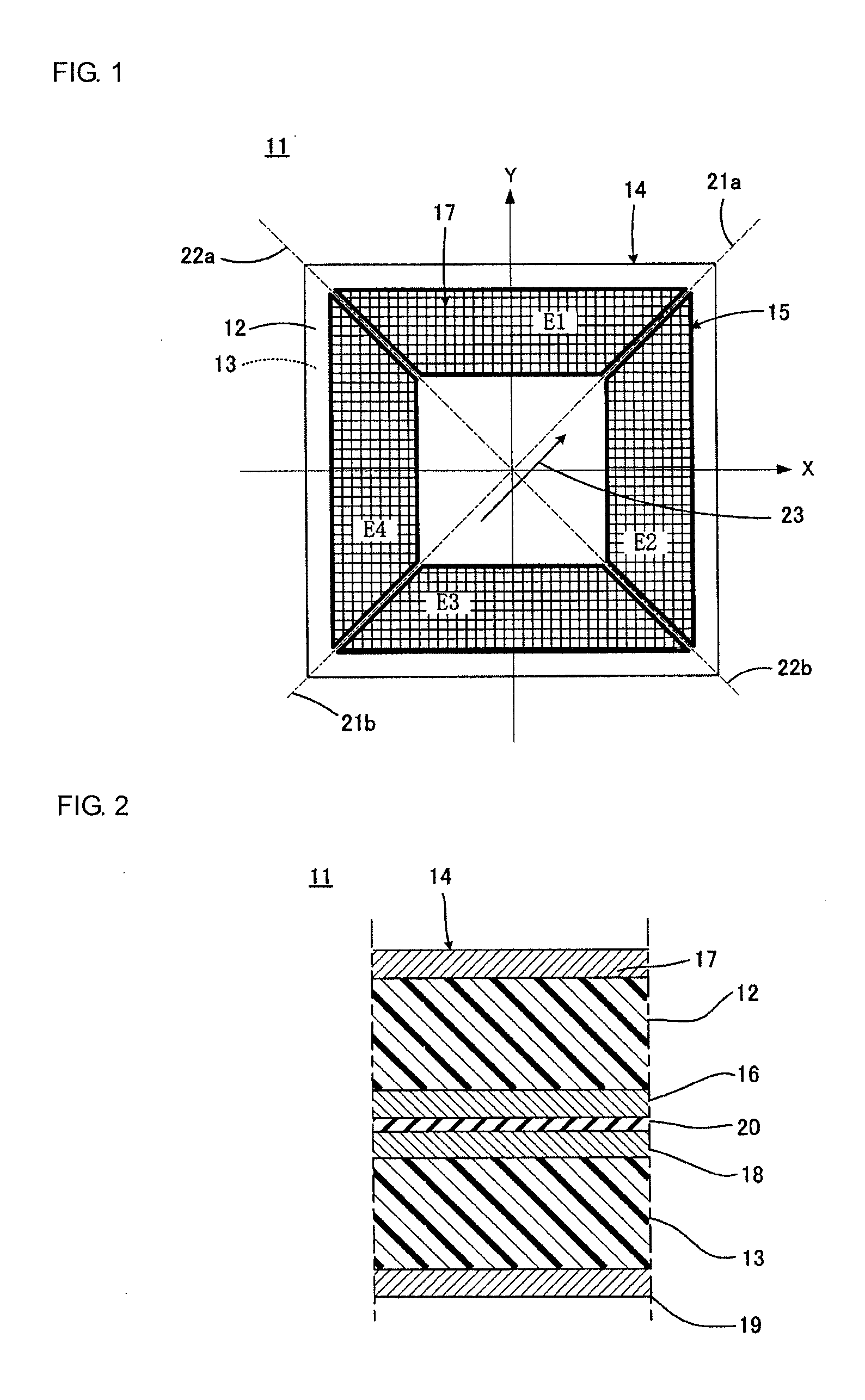
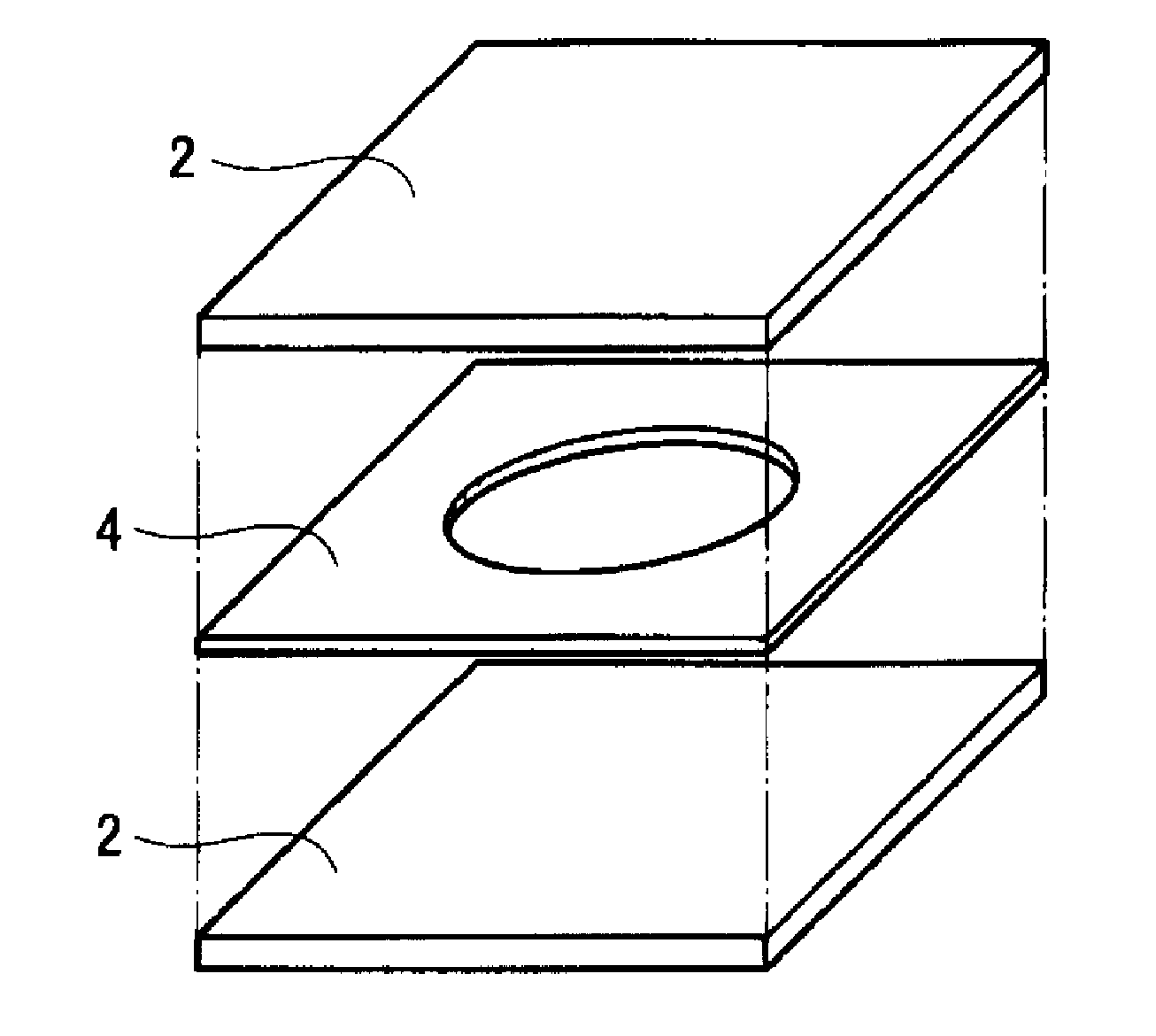
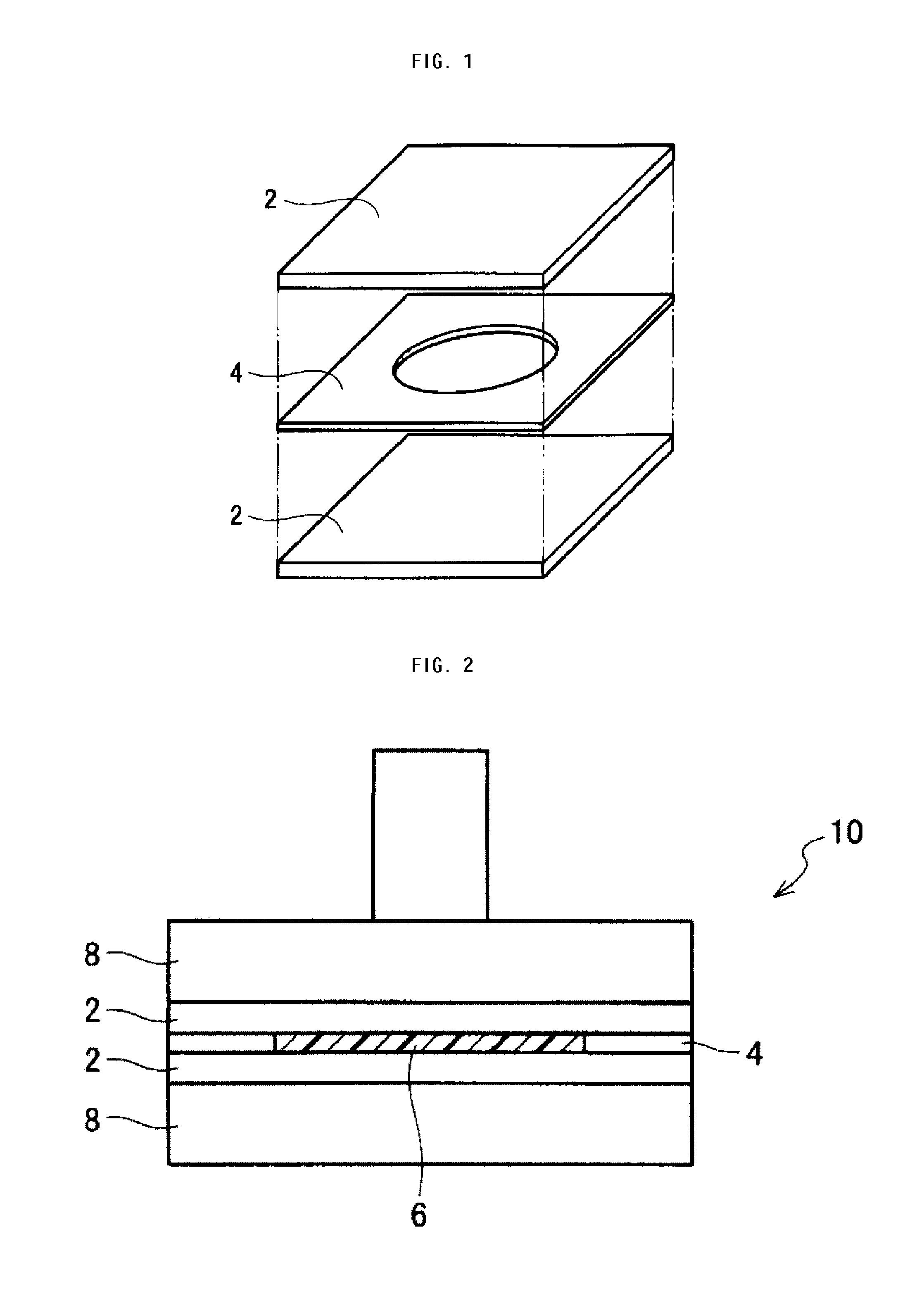
ActiveUS20120025674A1High piezoelectric constant dHigh transparencyPiezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostriction/magnetostriction machinesChiral polymersCrystallinity
Owner:MITSUI CHEM INC +1
Piezoelectric speaker, speaker apparatus, and tactile feedback apparatus
ActiveUS20110128245A1High transparencyImprove conductivityPiezoelectric/electrostrictive/magnetostrictive devicesTransducer casings/cabinets/supportsEngineeringLoudspeaker
A piezoelectric speaker that includes an electrode formed on opposing main surfaces of a piezoelectric sheet having a predetermined stretching axis and made of a chiral polymer. The electrode is divided into four electrode portions by a plurality of dividing lines extending in a radiation direction. The four electrode portions are distributed along the outer peripheral portion except for the central portion of a vibration region. Voltage is applied to the respective four electrode portions in such a manner that electric field vectors generated in the thickness direction of the piezoelectric sheet direct in opposite directions between the adjacent ones of four sheet portions of the piezoelectric sheet to which voltage is applied by the electrode portions.
Owner:MURATA MFG CO LTD
Chiral polymeric salen catalyst, and a process for preparing chiral compounds from racemic epoxides by using them
InactiveUS6903043B2Avoid deactivationEasy to produceRuthenium organic compoundsOrganic compound preparationDiolHydrolysis
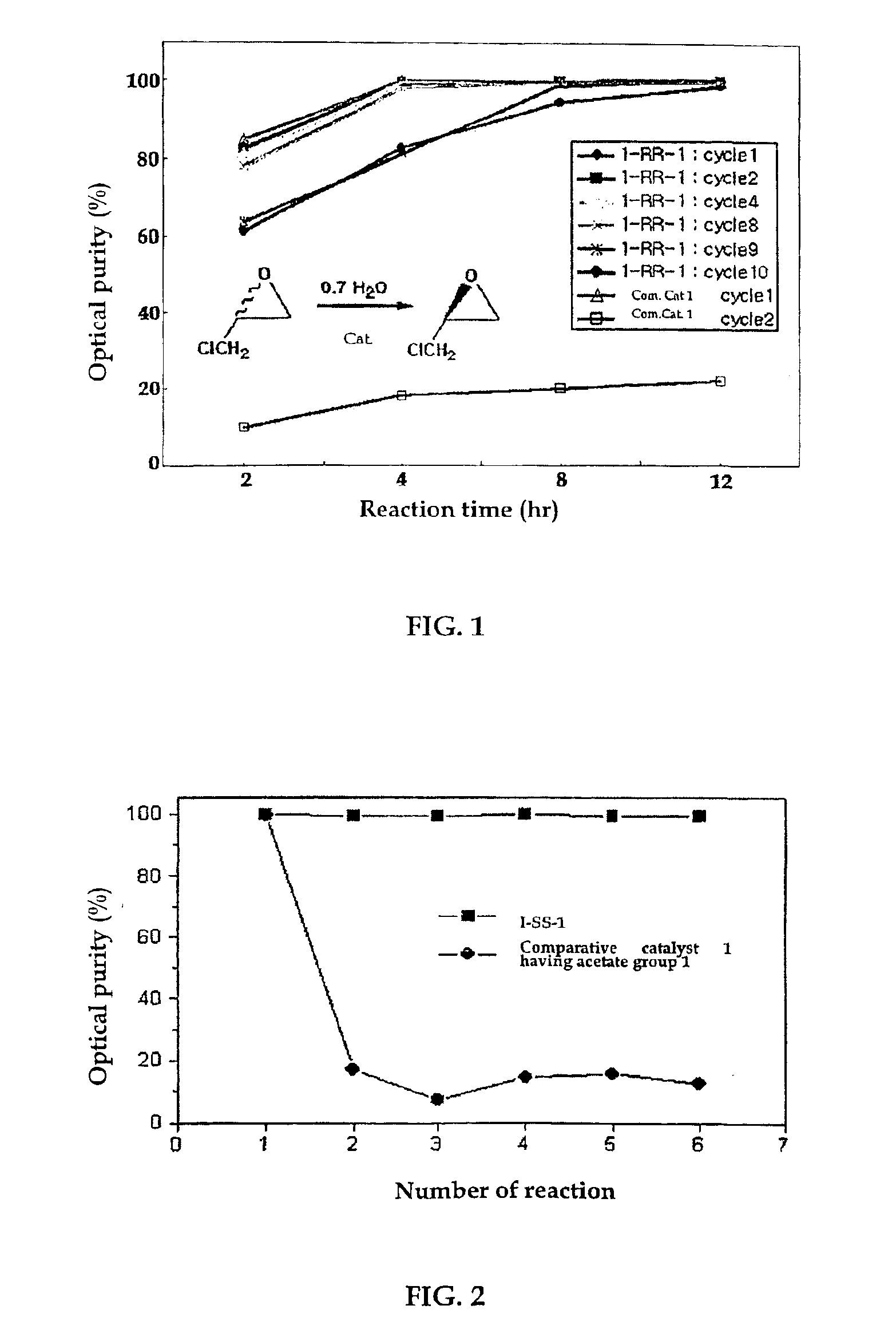
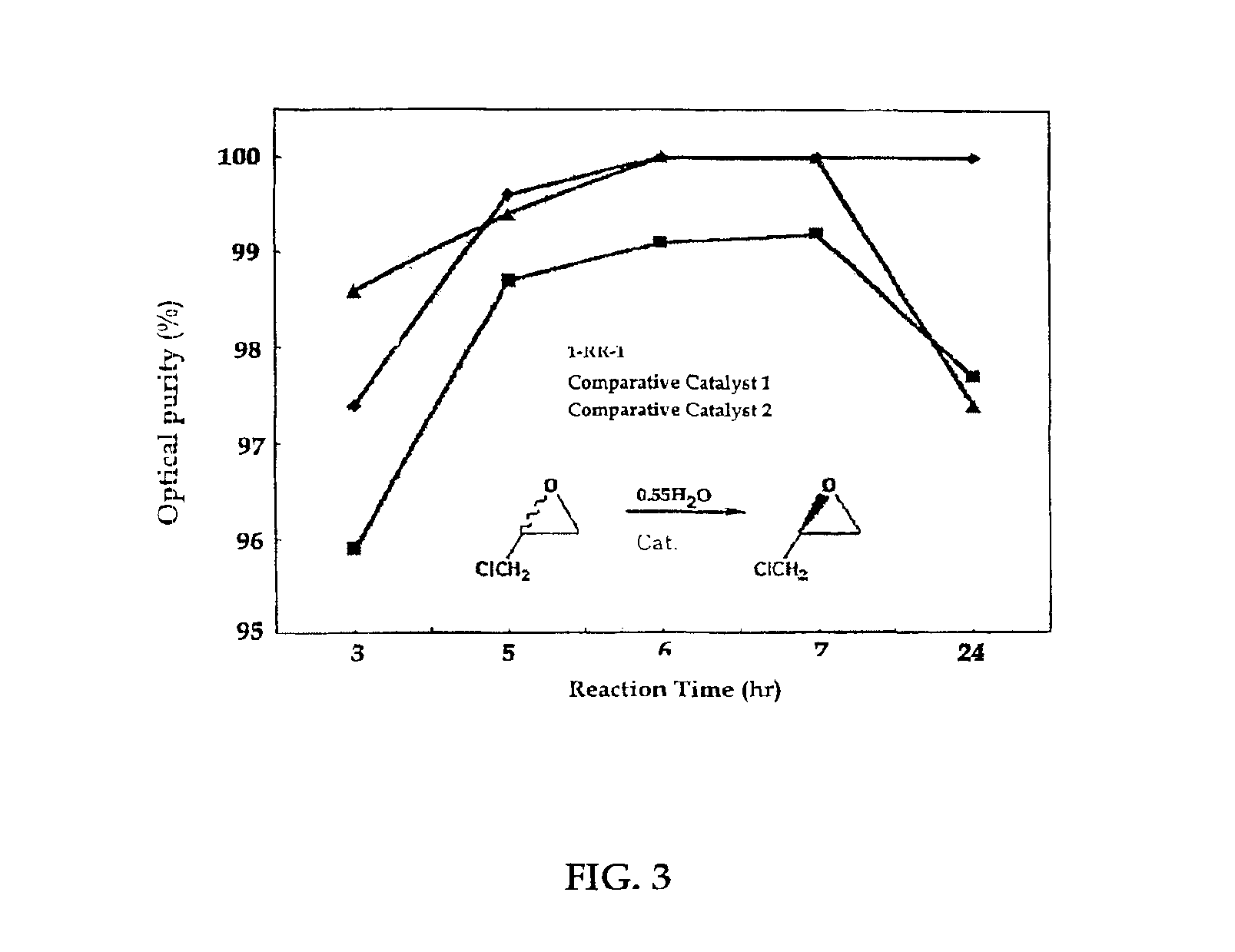
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide a chiral polymeric salen catalyst and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis or racemic epoxides.
Owner:RSTECH CO LTD
Preparation method of chiral fluorescent nanoparticle based on hyperbranched conjugated polymer
InactiveCN102627776AOvercome high pricesOvercoming complexityLuminescent compositionsSolubilityLimonene
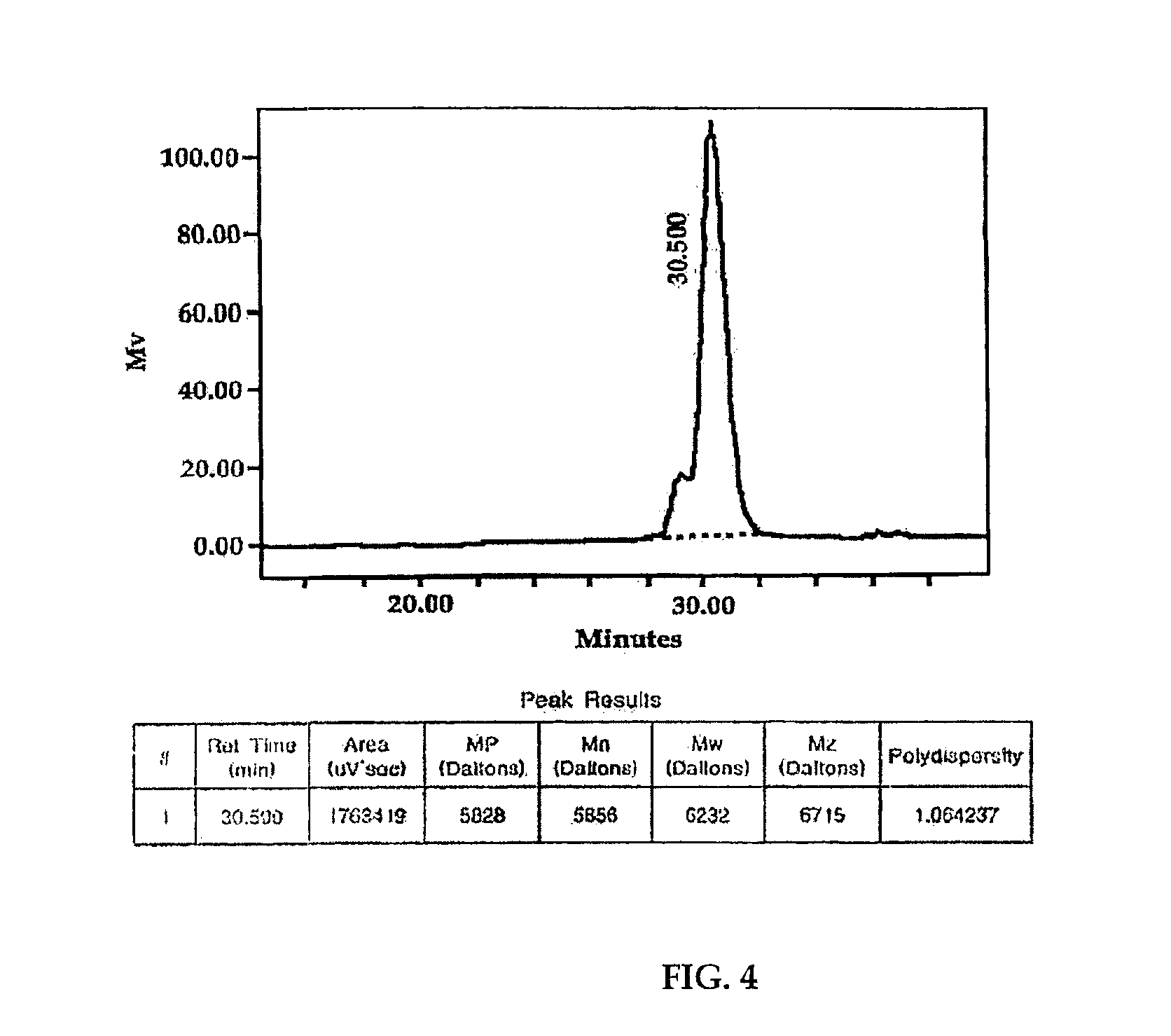
The invention discloses a preparation method of a chiral fluorescent nanoparticle based on a hyperbranched conjugated polymer. The method is characterized by comprising the following steps of: preparing a hyperbranched polymer containing 9,9-dioctylfluorene and a bithiophene unit into a trichloromethane solution of which the solubility is 1*10<-3> to 1*10<-1> mg / mL; and adding one of (R)-limonene or (S)-limonene into the trichloromethane solution of the copolymer together with methanol, and mixing uniformly to obtain a stable turbid solution, wherein the volume ratio of the (R)-limonene or (S)-limonene to the methanol to the copolymer is (1-8):(8-1):1. In the method, a solvent chiral transfer technology is applied to the preparation of a hyperbranched polymer chiral fluorescent nanoparticle with a hyperbranched polymer, so that the problems of high price of a chiral reagent, complex synthesis steps and the like existing in the conventional method for synthesizing the hyperbranched polymer chiral fluorescent nanoparticle are solved.
Owner:SUZHOU UNIV
Piezoelectric polymer material and method for producing same
ActiveUS20120132846A1High constantExcellent transparency and dimensional stabilityPiezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostrictive device material selectionCrystallinityMicrowave transmission
The invention provides a piezoelectric polymer material comprising a helical chiral polymer having a weight average molecular weight of from 50,000 to 1,000,000 and optical activity, the piezoelectric polymer material having: crystallinity as obtained by a DSC method of from 20% to 80%; a transmission haze with respect to visible light of 50% or less; and a product of the crystallinity and a standardized molecular orientation MORc, which is measured with a microwave transmission-type molecular orientation meter at a reference thickness of 50 μm, of from 40 to 700.
Owner:MITSUI CHEM INC +1
Infrared reflection device based on electrical response
ActiveCN105676489AReduce concentration differenceFacilitate transmissionNon-linear opticsElectricityUltraviolet lights
The present invention discloses an infrared reflection device based on electrical response and a preparation method thereof. Chiral doping agent, chiral monomers, photoinitiator, ultraviolet absorbent are mixed with negative liquid crystal to obtain a liquid crystal mixture, the liquid crystal mixture is disposed between two light transmission substrates to which voltage can be accessed, ultraviolet light irradiates a liquid crystal cell from one side of the first light transmission substrate, the photoinitiator drives the chiral monomers to polymerize a chiral polymer network under the effect of the ultraviolet light, the generated chiral polymer network concentration has a concentration gradient, namely the chiral monomer concentration has a concentration gradient, so that a pitch gradient of a negative liquid crystal helical structure is formed, and a wide bandwidth for reflecting infrared light can be obtained. The chiral polymer network can capture impurity cations in the liquid crystal mixture, when the substrates are powered, the impurity cations drive the chiral polymer network to move, so that the chiral monomer concentration gradient is decreased, the pitch gradient is decreased, and the purpose of changing the bandwidth from wide to narrow is achieved.
Owner:SHENZHEN GUOHUA OPTOELECTRONICS +2
Applications of chiral polymer catalyst in asymmetric reaction
ActiveCN106853380AThe synthesis method is simpleShort reaction timeOrganic compound preparationOrganic chemistry methodsDispersityPorosity
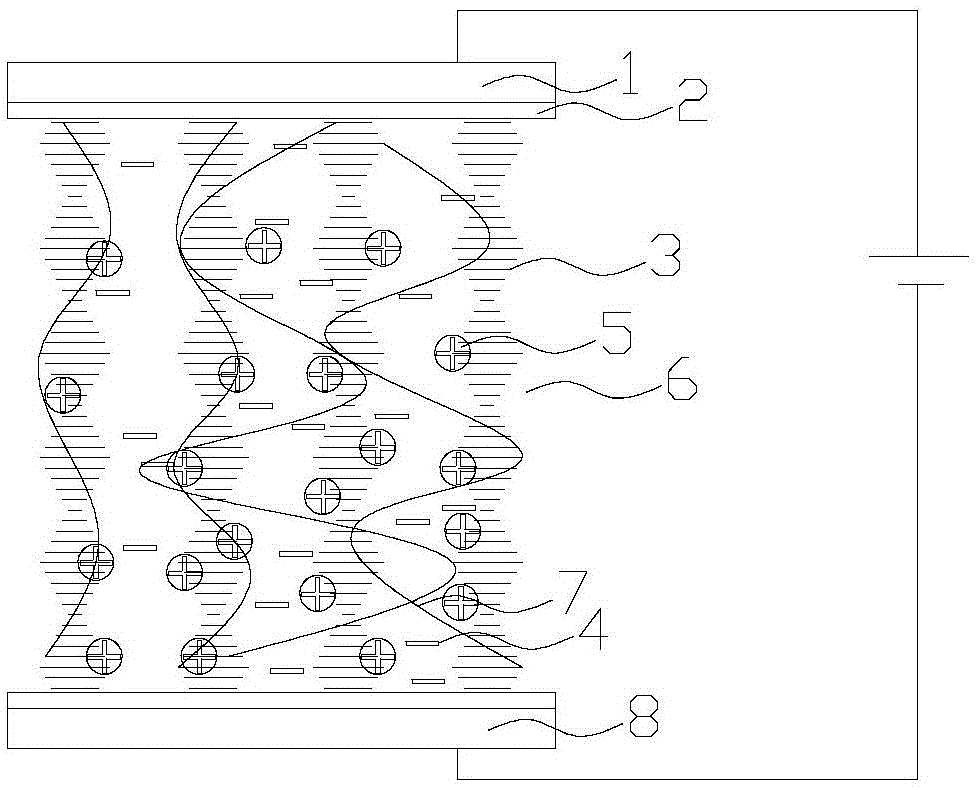
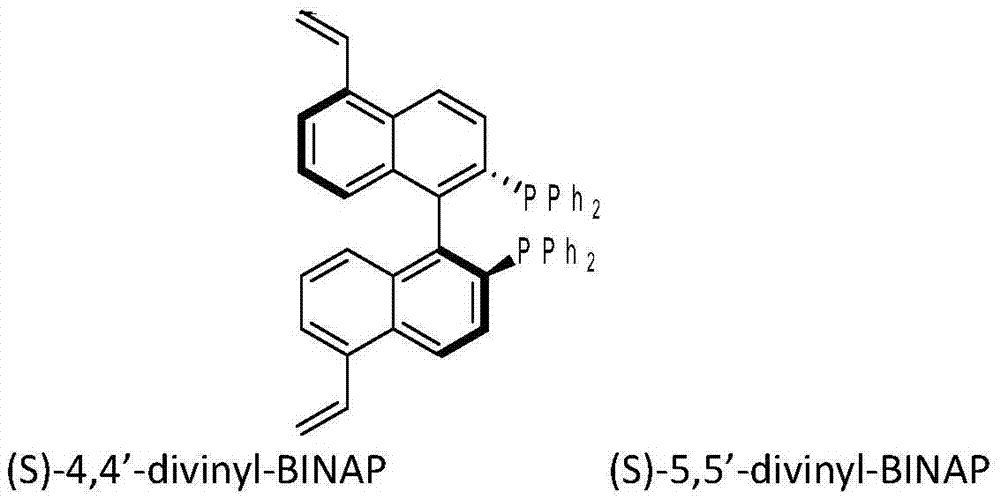
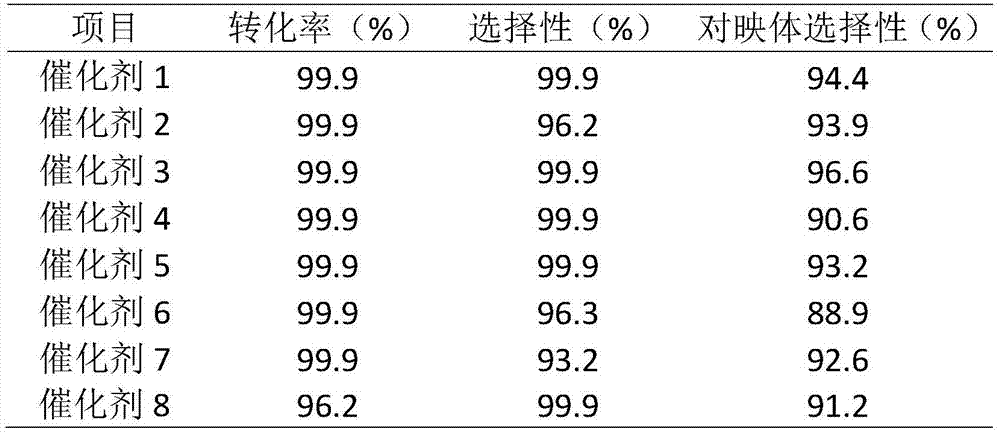
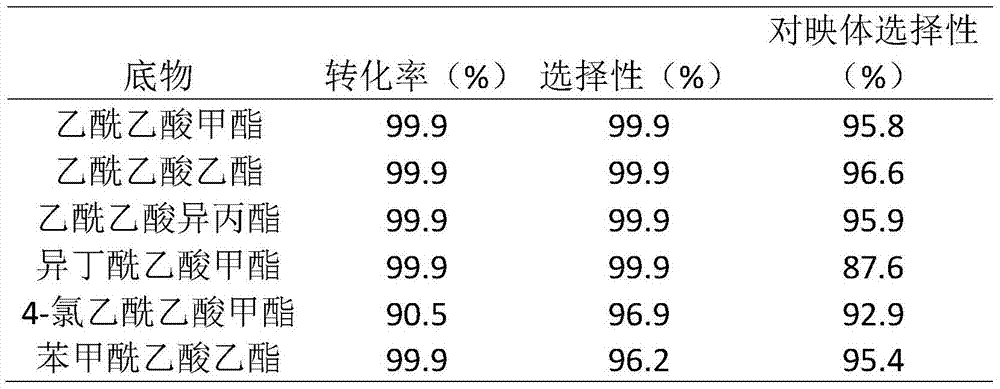
The invention discloses applications of a chiral polymer catalyst in asymmetric reaction, and belongs to the field of material synthesis and application. The phosphine-containing polymer is obtained via mixed polymerization of vinyl-containing chiral bidentate phosphine ligand BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) and derivative of vinyl-containing chiral bidentate phosphine ligand BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) with other vinyl comonomers. The vinyl polymer possesses relatively large specific surface area and porosity, and excellent thermal stability and chemical stability. In the heterogeneous catalyst, one or a plurality of elements selected from Ru, Rh, Ir, Pa, Au, and Cu are taken as active ingredients. The chiral ligand is uniformly embedded into and highly dispersed in a polymer skeleton, so that metal dispersion degree on the catalyst is relatively high, and relatively high catalytic activity is achieved. The heterogeneous catalyst is suitable for a plurality of reaction technology including intermittent still reaction, continuous fixed bed reaction, and trickle bed reaction. When the heterogeneous catalyst is used in catalytic kettle-type asymmetric hydrogenation, high target product yield is achieved, and enantioselectivity is higher than 96%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Piezoelectric speaker, speaker apparatus, and tactile feedback apparatus
ActiveUS8363863B2Increase freedomOptimizationPiezoelectric/electrostrictive/magnetostrictive devicesTransducer casings/cabinets/supportsElectricitySplit lines
A piezoelectric speaker that includes an electrode formed on opposing main surfaces of a piezoelectric sheet having a predetermined stretching axis and made of a chiral polymer. The electrode is divided into four electrode portions by a plurality of dividing lines extending in a radiation direction. The four electrode portions are distributed along the outer peripheral portion except for the central portion of a vibration region. Voltage is applied to the respective four electrode portions in such a manner that electric field vectors generated in the thickness direction of the piezoelectric sheet direct in opposite directions between the adjacent ones of four sheet portions of the piezoelectric sheet to which voltage is applied by the electrode portions.
Owner:MURATA MFG CO LTD
Load type chiral catalyst and preparation method thereof
InactiveCN102909070AHigh activityGood dispersionOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesStructural formulaSilicon dioxide
The invention discloses a load type chiral catalyst and a preparation method thereof. The load type chiral catalyst is characterized in that the load type chiral catalyst is proline-loaded hair-shaped silica nano particle obtained by forming a functional group on the surface of nano silica in a finishing manner, triggering chiral N-p-vinyltoluene sulfonyl-Boc-L-proline amide to polymerize by an reversible-breakage-chain transfer polymerization method, grafting a chiral polymer chain on the surface of the nano silica and further deprotecting, and the structural formula of the load type chiral catalyst is shown as follow. The invention discloses the preparation method of the load type chiral catalyst. The load type chiral catalyst can realize the advantages of separability and recyclability besides being capable of obtaining high-stereoselectivity chiral products in asymmetric catalyzing reaction, and production cost is reduced.
Owner:HENAN NORMAL UNIV
Sensor device and electronic apparatus
ActiveCN104321613AImprove detection efficiencyNo pyroelectricityForce measurement using piezo-electric devicesElectrical/magnetic solid deformation measurementEngineeringShearing deformation
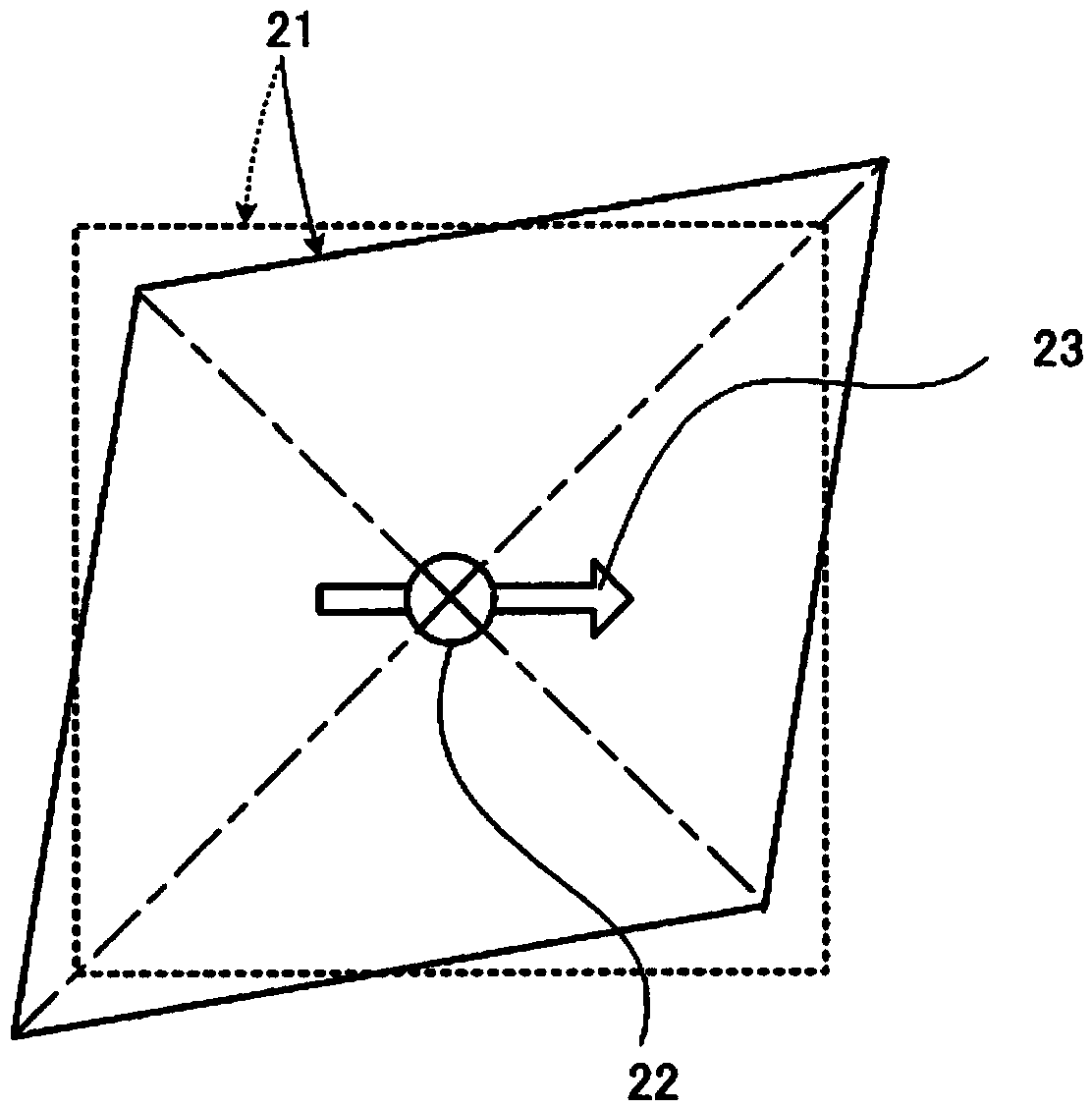
Provided is a sensor device which employs a piezoelectric film, formed from a chiral polymer such as polylactic acid, in sensing a displacement. A sensor device comprises: a film (21) formed from, for example, PLLA; and electrodes for extracting an output voltage from the PLLA film (21), and which are formed on both primary surfaces of the PLLA film (21) in a state of mutually facing one another and sandwiching at least a portion of the PLLA film (21). A first edge (24) of the PLLA film (21) is fixed, and a second edge (25) which is opposite thereto is a movable part (28). The electrodes are made to extract an output voltage by an effect of a piezoelectric constant (d14) which is caused by a shear deformation which arises by a displacement of the movable part (28) in a parallel direction to the primary surfaces of the PLLA film (21), and is capable of sensing an operation caused by friction, etc.
Owner:MURATA MFG CO LTD
Security film and process for preparation thereof
ActiveUS20110293858A1High light transmittanceGood weather resistanceNon-fibrous pulp additionOther printing matterInformation layerPolymer resin
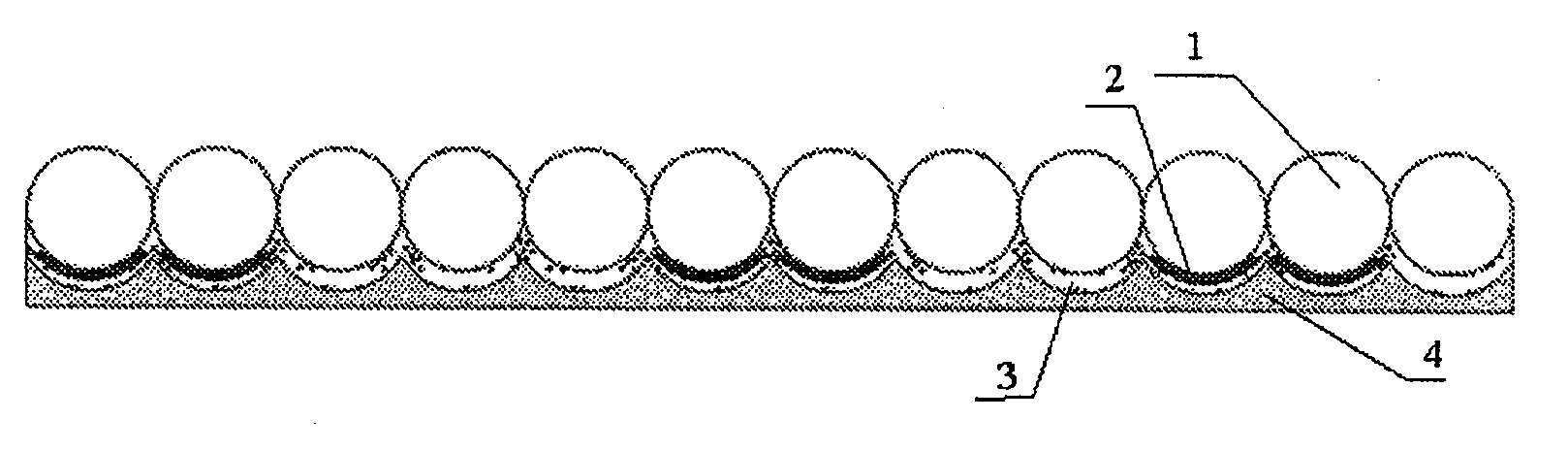
Disclosed herein are an anti-counterfeiting film and process for preparation thereof. The anti-counterfeiting film comprises a directional retroreflective layer (1), an optical-angle-based color-changing information layer (2), a reflective layer (3) and a support layer (4) which are combined in this turn. The support layer (4) is made of a polymer resin material, the reflective layer (3) is made of a material with high reflectivity and has a thickness of nanoscale, the information layer (2) is made of an optical-angle-based color-changing chiral polymer material having a helical structure or made of an optical-angle-based color-changing cellulose material. The optical-angle-based color-changing chiral polymer material having a helical structure is a condensation product of a chiral compound and a compound with functional group. The optical-angle-based color-changing cellulose material is composed of a cellulose derivative and a polymerisable monomer. The directional retroreflective layer (1) is composed of spheres which are made of optical material, and is spherically embedded in the support layer (4), wherein the refractive index of the material for the directional retroreflective layer (1) is 1.9-1.93.
Owner:SHANGHAI TECHSUN ANTI COUNTERFEITING TECH HLDG
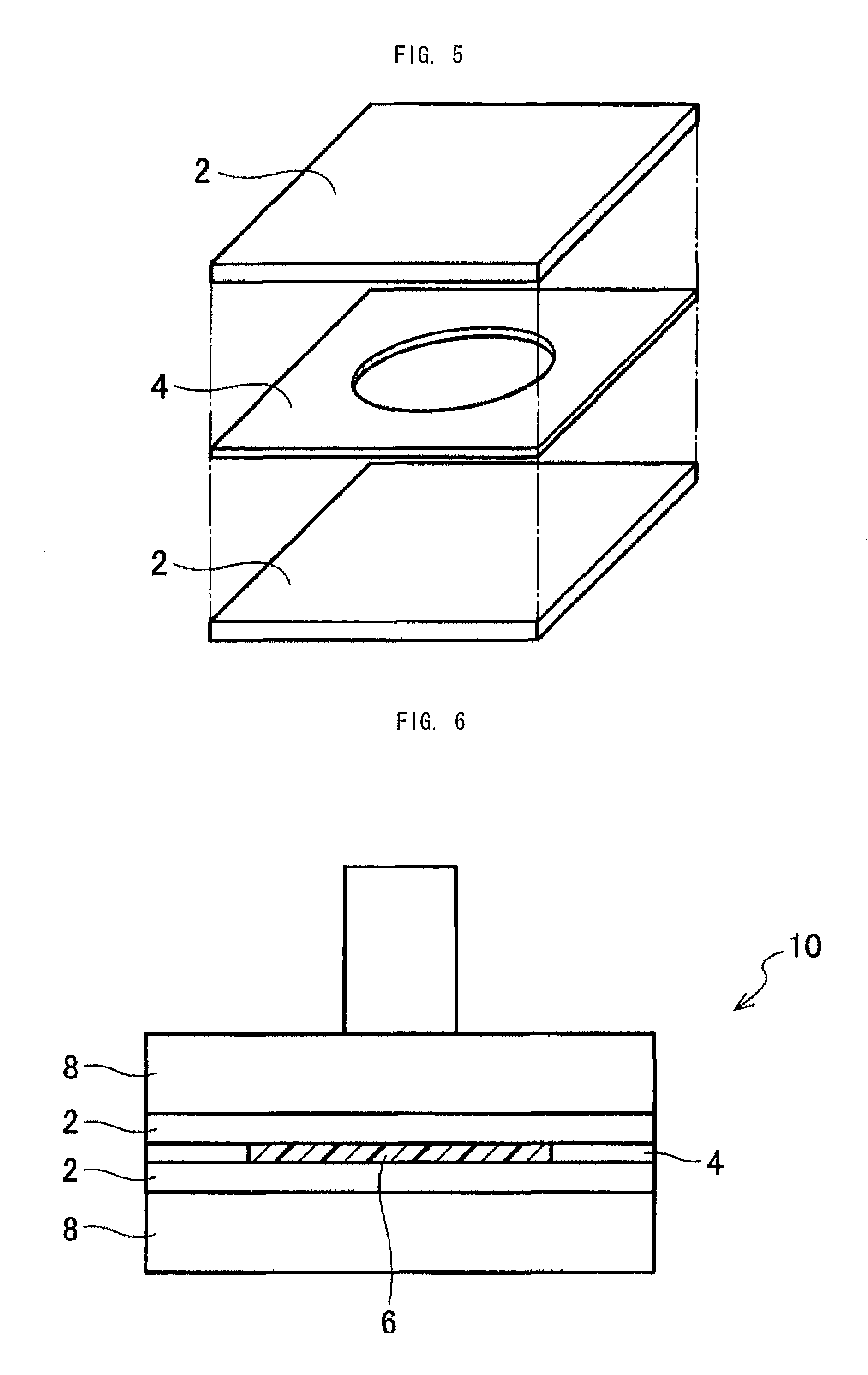
Piezoelectric polymer material and method for producing same
ActiveUS8829121B2High constantExcellent transparency and dimensional stabilityPiezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostrictive device material selectionCrystallinityMicrowave transmission
The invention provides a piezoelectric polymer material comprising a helical chiral polymer having a weight average molecular weight of from 50,000 to 1,000,000 and optical activity, the piezoelectric polymer material having: crystallinity as obtained by a DSC method of from 20% to 80%; a transmission haze with respect to visible light of 50% or less; and a product of the crystallinity and a standardized molecular orientation MORc, which is measured with a microwave transmission-type molecular orientation meter at a reference thickness of 50 μm, of from 40 to 700.
Owner:MITSUI CHEM INC +1
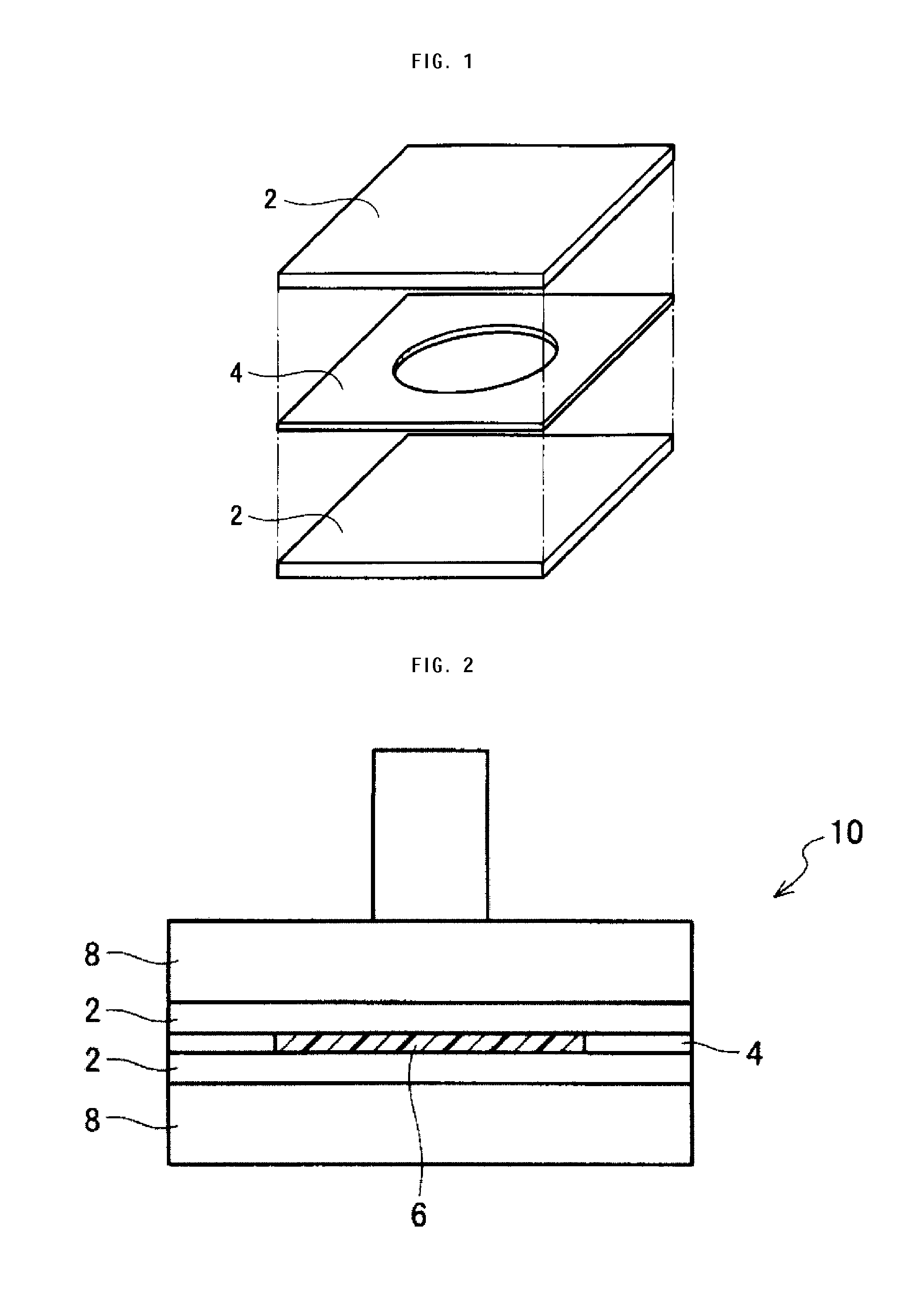
Piezoelectric polymer material, process for producing same, and piezoelectric element
ActiveUS8648151B2High constantHigh transparencyPiezoelectric/electrostrictive device manufacture/assemblyCeramic shaping apparatusPolymer scienceX-ray
The invention provides a piezoelectric polymer material including a helical chiral polymer having a weight average molecular weight of from 50,000 to 1,000,000 and having optical activity, the piezoelectric polymer material having a piezoelectric constant d14 at 25° C. of 10 pC / N or more, a degree of crystallinity obtained by X-ray diffraction of from 40% to 80%, and a haze of from 0.5 to 30.
Owner:MITSUI CHEM INC +1
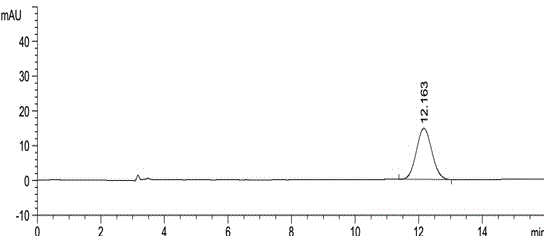
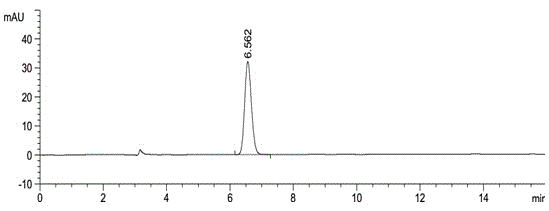
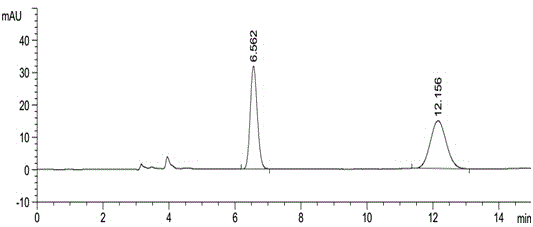
Method for using liquid chromatography to separate and measure apremilast and enantiomer thereof
ActiveCN104820028AAccurate quality controlAccurate separation detectionComponent separationAlkaneEnantiomer
The invention relates to a method for using a liquid chromatography to separate and measure apremilast and enantiomer thereof. The method includes that using spherical silica gel coated with chiral polymer at surface as a chiral chromatographic column, using alkane-different concentrations of polarity organic solvent as mobile phase, wherein the polarity organic solvent is composed of first organic solvent and second organic solvent, the alkane is selected from normal hexane, normal heptane, cyclohexane and methylene dichloride, the first organic solvent is isopropanol, and the second organic solvent is selected from methanol, ethanol and acetonitrile. The method for using the liquid chromatography to separate and measure the apremilast and enantiomer thereof solves the problem that the apremilast and enantiomer thereof are difficult to separate for the separation and measurement, and accordingly the controllable quality of the apremilast and the preparation thereof is guaranteed.
Owner:CHONGQING PHARMA RES INST
Polymer organocatalyst and preparation process
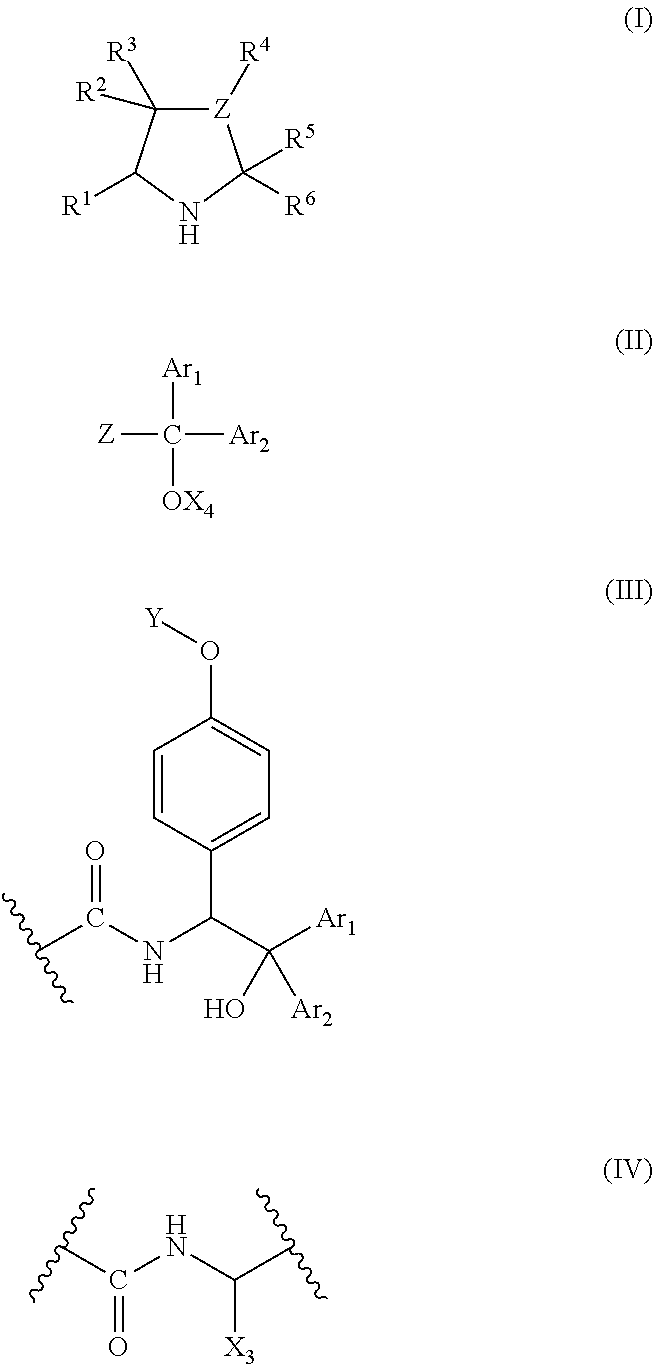
A chiral polymer organocatalyst comprising a main chain and side chain organocatalytic groups covalently attached to the main chain, which organocatalytic groups comprise an amino acid or amino acid derivative of the following general formula (I), in which one stereoisomeric form predominates: formula (I) wherein the catalyst is bound to the polymer main chain via R1, R2, R4, R5 or R6 through a linker (L) or direct bond, and wherein R1-R6 and Z are defined as follows: R1 is H, a naturally occurring alpha-amino acid side chain or a non-natural commercially available alpha-amino acid side chain that may contain L; R2 is H, O (doubly bonded to give a carbonyl), O-L (where L is a linker), NH-L or L; R3 is H or doubly bonded to give a carbonyl with R2 when R2 is O; R4 is H, C1-C6 alkyl or L R5 is H, CO2H, C1-C6 alkyl, benzyl, L, CONHR (in which R is alkyl, aryl, heteroaryl, arylalkyl or, heteroarylalkyl), tetrazolyl, CH2 coupled to a triazole moiety, an esterified CH2OH or CO2R (in which R is alkyl, aryl, heteroaryl, arylalkyl N or heteroarylalkyl), formula (II) or formula (III) wherein z is formula (IV) or a directed bond, X4 is H, Me3Si or Et3Si, X3 comprises a naturally-occurring alpha-amino acid side chain, H, C1-C6 alkyl or phenyl, Ar1 and Ar2 are each independently aryl or heteroaryl, and Y denotes the position of attachment to the main chain or linker; and R6 is H, CO2H3 C1-C6 alkyl, benzyl or L; and wherein the polymer organocatalyst comprises a cross-linked polymer.
Owner:UNIVERSITY OF OSLO
Temperature response type chiral polymer hydrosol with branched chain structure
InactiveCN102627723ACapable of chiral recognitionEasy to useOther chemical processesAlkali metal oxides/hydroxidesTemperature responseCross-link
The invention discloses a temperature response type chiral polymer hydrosol with a branched chain structure and a preparation method thereof. The hydrosol consists of chiral monomer M1 containing an L-amino acid group and a flexible branched chain with two to six carbon atoms and alkyl acrylamide M2 with temperature sensitive characteristic, wherein the mass percent M1:M2 of using amounts of the M1 and M2 is equal to 5-25:95-75. The preparation method comprises the following steps of: esterifying by taking the L-amino acid as a raw material; condensing with acrylic acid to prepare the chiral monomer M1; and dissolving M1 and M2 in absolute ethanol under an oxygen-free condition to perform free radical copolymerization reaction to obtain the temperature response type chiral polymer hydrosol. The temperature response type chiral polymer hydrosol has the advantages of simple process, low cost, convenience for industrial promotion and application and the like; the temperature sensitivity and the chiral separation efficiency of the hydrosol can be adjusted by changing the temperature, the rate of charge, the using amount of a cross-linking agent and raw materials and can be used for separation research of a chiral compound.
Owner:TIANJIN POLYTECHNIC UNIV
Chiral Polymers for the Self-Assembly of Photonic Crystals
Owner:CALIFORNIA INST OF TECH
Asymmetric anionic copolymerization method of methacrylate chiral polymer
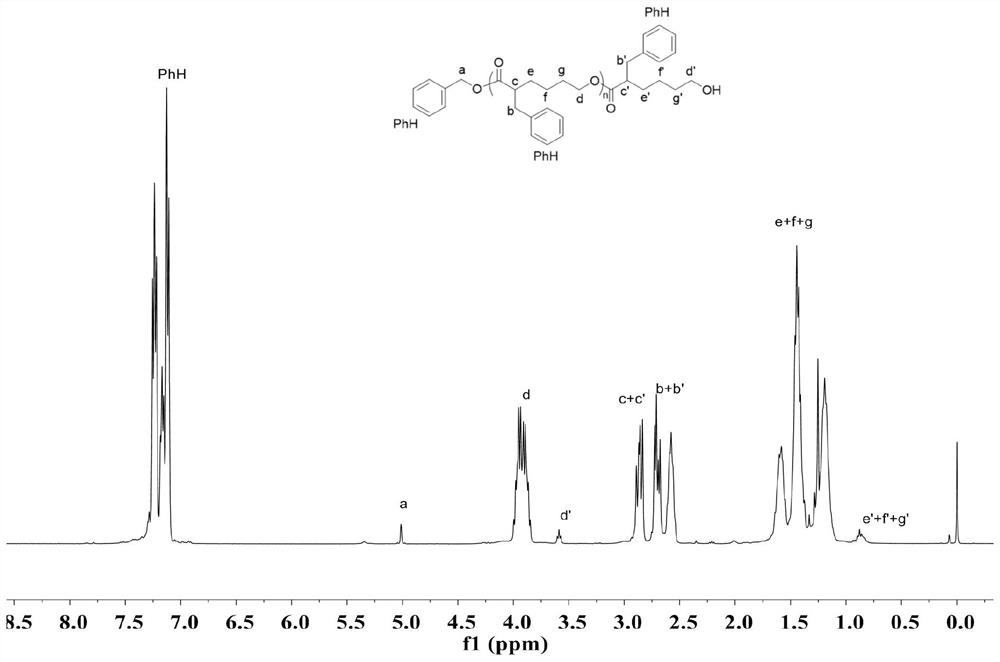
The invention provides an asymmetric anionic copolymerization method of a methacrylate chiral polymer. The method comprises the following steps: reacting methacrylic acid with trityl chloride to generate a large-volume triphenylmethyl acrylate monomer, carrying out an asymmetric anionic copolymerization reaction on the large-volume triphenylmethyl acrylate monomer and a self-made chiral function monomer under specific anion conditions to obtain a large-volume methacrylate chiral polymer; and purifying the polymer to finally obtain a target product. The structural characterization and analysis of the obtained polymer are carried out by using nuclear magnetic resonance hydrogen spectrum (<1>H-NMR) and an element analysis technology to determine that the molecular structure of the polymer and proportions of all components of the polymer accord with design requirements. Performances of the synthesized copolymer are deeply analyzed through using a gel permeation chromatograph and a polarimeter to obtain the molecular weight of the novel chiral copolymer and the distribution thereof, and the optical active characteristics of the copolymer. The method has the advantages of clear and feasible synthesis route, manure process, simple operation, easy realization, and large-scale batch production.
Owner:HARBIN ENG UNIV
Chiral polymer microsphere with porous structure and preparation method thereof
ActiveCN110964142AIncrease the chiral interaction pointImprove the effect of chiral separationLiquid crystal compositionsOther chemical processesChromatographic separationStationary phase
Owner:JIANGSU JICUI INTELLIGENT LCD TECH CO LTD
Yellowish green luminous chiral polymer crystal material
The invention relates to a yellowish green luminous crystal material. The chemical formula of the crystal material is [Zn(H2O) (ONCP) Cl]n; the crystal material belongs to a monoclinic crystal system; a space group is P21; beta is equal to 91.92 (2) degrees; alpha and gamma are equal to 90 degrees; Z is equal to 2; and [Zn(H2O) (ONCP) Cl]n monocrystal is synthesized by a hydrothermal method. The crystal is obtained by performing a hydrothermal reaction on ZnCl2 and a ligand HONCP which serve as reaction raw materials at the temperature of 140 DEG C, preserving heat for three days and slowly cooling to room temperature. The crystal material can be applied to the research and development of white light-emitting diodes.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
New typed sugar of C12 high carbon and ramification, preparation method and application
InactiveCN1660852AEnhance anti-inflammatoryHigh activitySugar derivativesNitro compoundHydroxylamine
A high-carbon C12 saccharide compound and its derivatives are prepared through dissolving 1,4,3,6-dianhydropectose in organic solvent, and catalytic addition reaction. It can be used to synthesize chiral aminoalcohol, aminosaccharide, oxazoline, etc, the chiral polymers, and amino derivatives.
Owner:KAIFENG PHARMA GRP
Method for constructing porous polymer on the basis of pure chiral molecules of 1, 1 '-bi-2-naphthol
ActiveCN106939059AGood chiral induction environmentLarge specific surface areaGroup 5/15 element organic compoundsSynthesis methods2-Naphthol
Owner:ZHEJIANG UNIV
Alkoxy ether thermosensitive chiral polymer nano-microspheres and preparation method thereof
The invention relates to alkoxy ether thermosensitive chiral polymer nano-microspheres and a preparation method thereof. The nano-microspheres are prepared by carrying out copolymerization reaction on alkoxy ether dendronized macromonomer with chiral induction primitive and p-alkynyl benzaldehyde to obtain a copolymer, and cross-linking the copolymer by using adipic dihydrazide as a cross-linking agent to form the alkoxy ether chiral polymer nano-microspheres, wherein the mol ratio of the aldehyde group in the copolymer to the cross-linking agent is 2:1. The nano-microspheres have good dispersibility in water solution and excellent thermosensitive behavior, and by adjusting temperature, concentration and molecular weight, chiral nano-microspheres with different particle sizes can be prepared. Simultaneously, compared with variable helical conformation of copolymers, the conformation of the obtained nano-microspheres is stable and single. The nano-microspheres are constructed by cross-linking a dynamic bond with a thermosensitive polymer, chiral transference of the chiral induction primitive provides a theoretical basis, and the nano-microspheres are of important significance in fields of realizing selectivity, identification or function control of some protein and amino acid, medicine release, envelop and the like.
Owner:SHANGHAI UNIV
Method for copolymerizing asymmetrical free-radicals of methacrylate chiral polymer
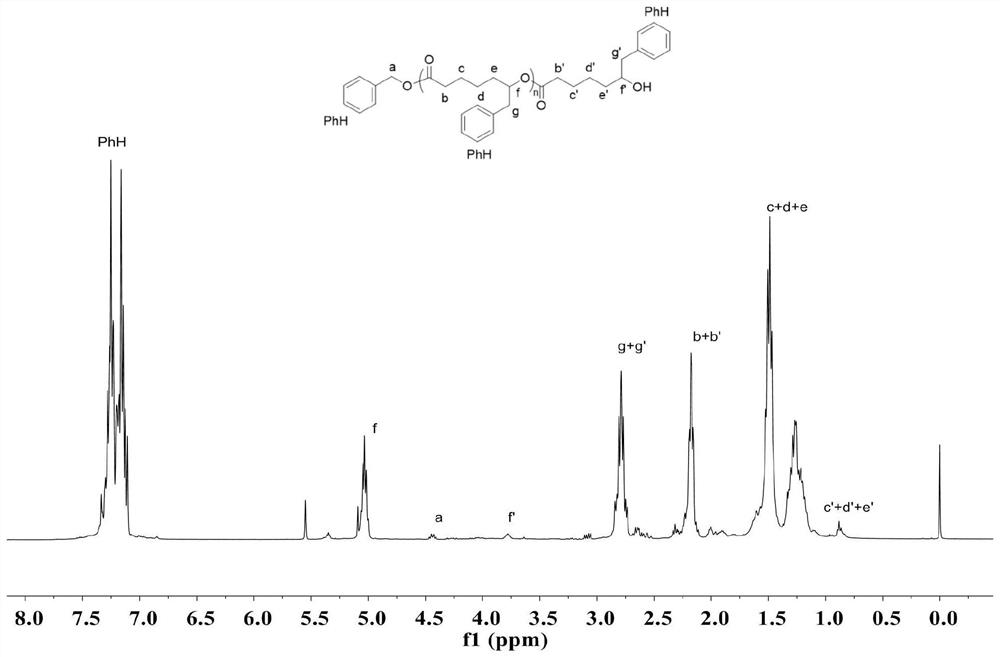
The present invention provides a method for asymmetrical free-radicals of methacrylate chiral polymer. The method comprises: firstly performing a reaction on methacrylic acid with trityl chloride to generate a methacrylic acid / trityl chloride monomer in a large volume; then performing asymmetrical free-radical copolymerization on the monomer and a chiral functional monomer under specific free-radical polymerization conditions; and obtaining a methacrylate chiral copolymer in a large volume; and performing purification on the obtained polymer to finally obtain a target product. NMR (1H-NMR) and elemental analytical techonlogies are are used for structural characterization and analysis of the obtained polymer to determine that the molecular structure and the component ratio meet design requirements. Gel permeation chromatography and a polarimeter are used for performing in-depth analysis on performances of the synthesized copolymer to obtain molecular weight and distribution of the new chiral copolymer and features of optical activity. The synthesis route is clear and feasible, the process is sophisticated, the operations are simple, and the method is easy to implement; and the method can be used for large-scale batch production.
Owner:HARBIN ENG UNIV
Supermolecular chiral porous polymer separation medium and preparation method and application thereof
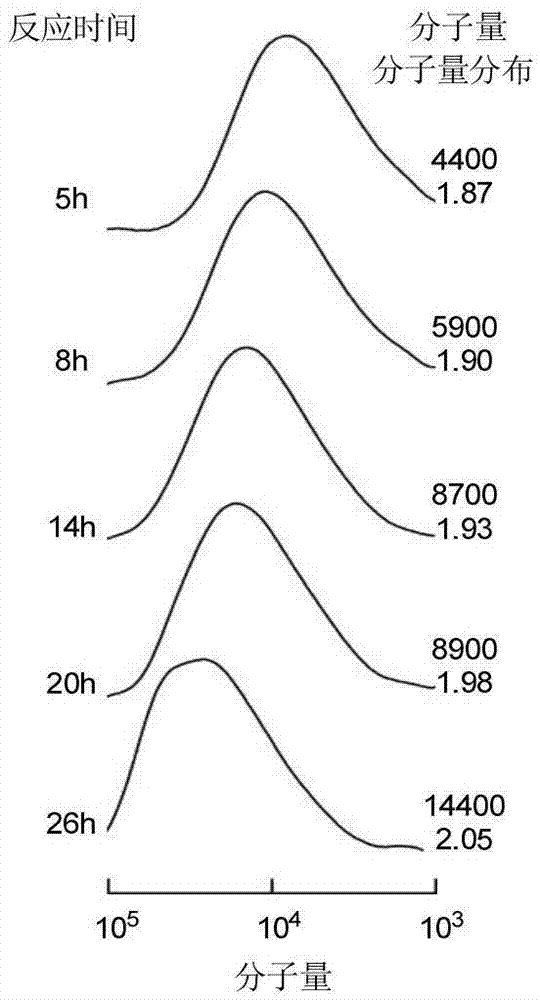
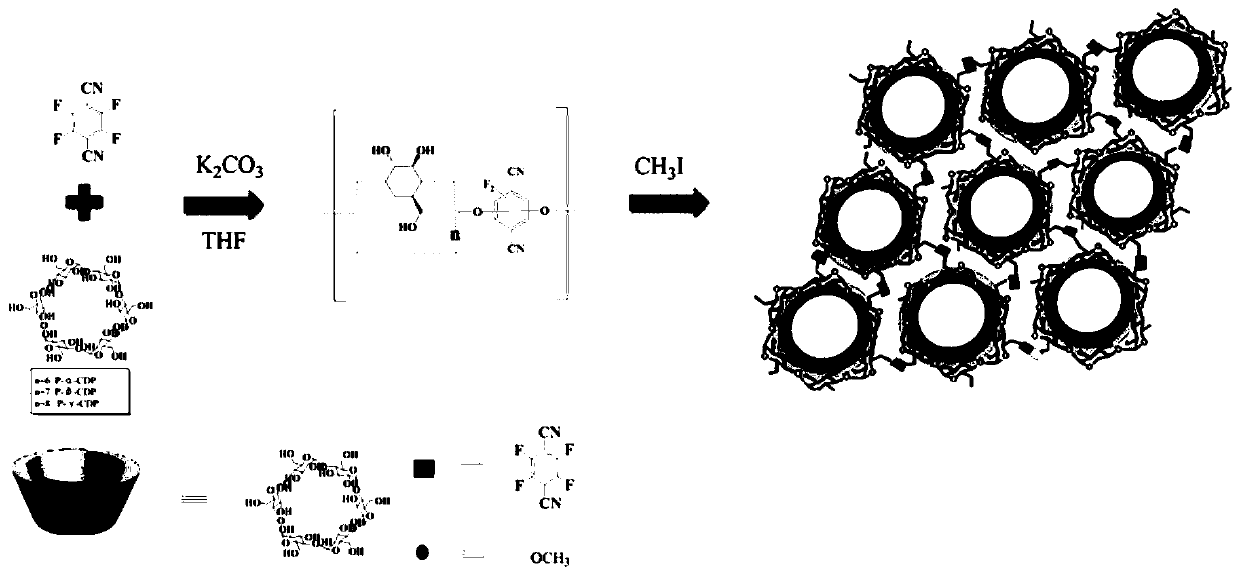
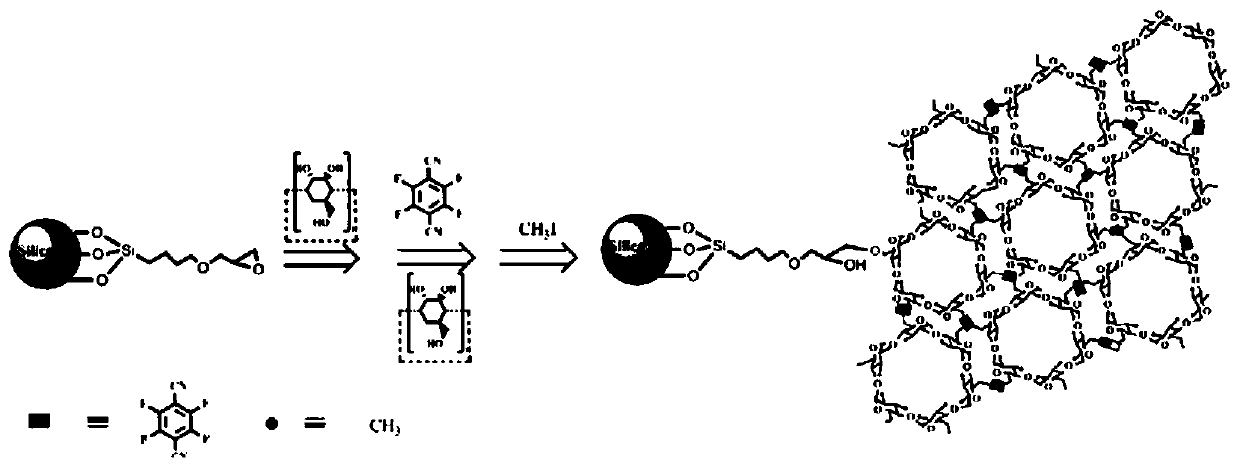
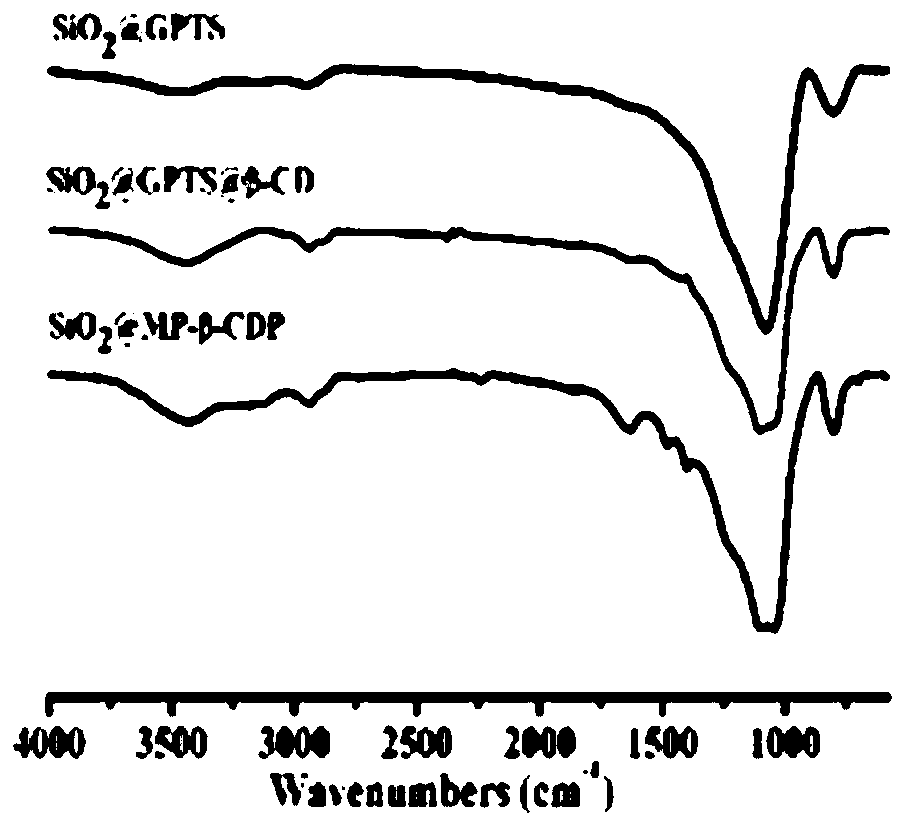
ActiveCN109762080AHigh chiral selectivityLarge adsorption capacityIon-exchange process apparatusIon-exchanger regenerationSorbentSynthesis methods
The invention discloses a supermolecular chiral porous polymer separation medium. A preparation method of the supermolecular chiral porous polymer separation medium comprises the following steps: in the presence of potassium carbonate, performing a reaction of cyclodextrin and a tetrafluoroterephthalonitrile monomer at a certain temperature; then introducing methyl by a post modification method toprepare a supermolecular chiral porous polymer adsorption material. According to the reaction, a supermolecular chiral porous polymer chromatographic stationary phase material is prepared by a layer-by-layer modification method. The chiral separation medium synthesized by adopting the method has the advantages of simple and convenient synthesis method, wide application range of the preparation method and the like. A cyclodextrin cavity structure in a porous polymer structure has various acting sites such as inclusion interaction, hydrogen-bond interaction and host-guest recognition. As a chiral chromatographic stationary phase and a chiral adsorbent, the supermolecular chiral porous polymer separation medium can recognize and separate certain chiral substances. The chiral separation medium has specific recognition and retaining acting force to certain polar chiral substances such as alcohols, aromatic carboxylic acids and aromatic amine.
Owner:SUN YAT SEN UNIV
A novel chiral polymer for enantioselective separation and process for preparation thereof
ActiveUS20180066102A1Hydroxy group formation/introductionCarboxylic acid ester formation/introductionAlcoholPolyfluorene
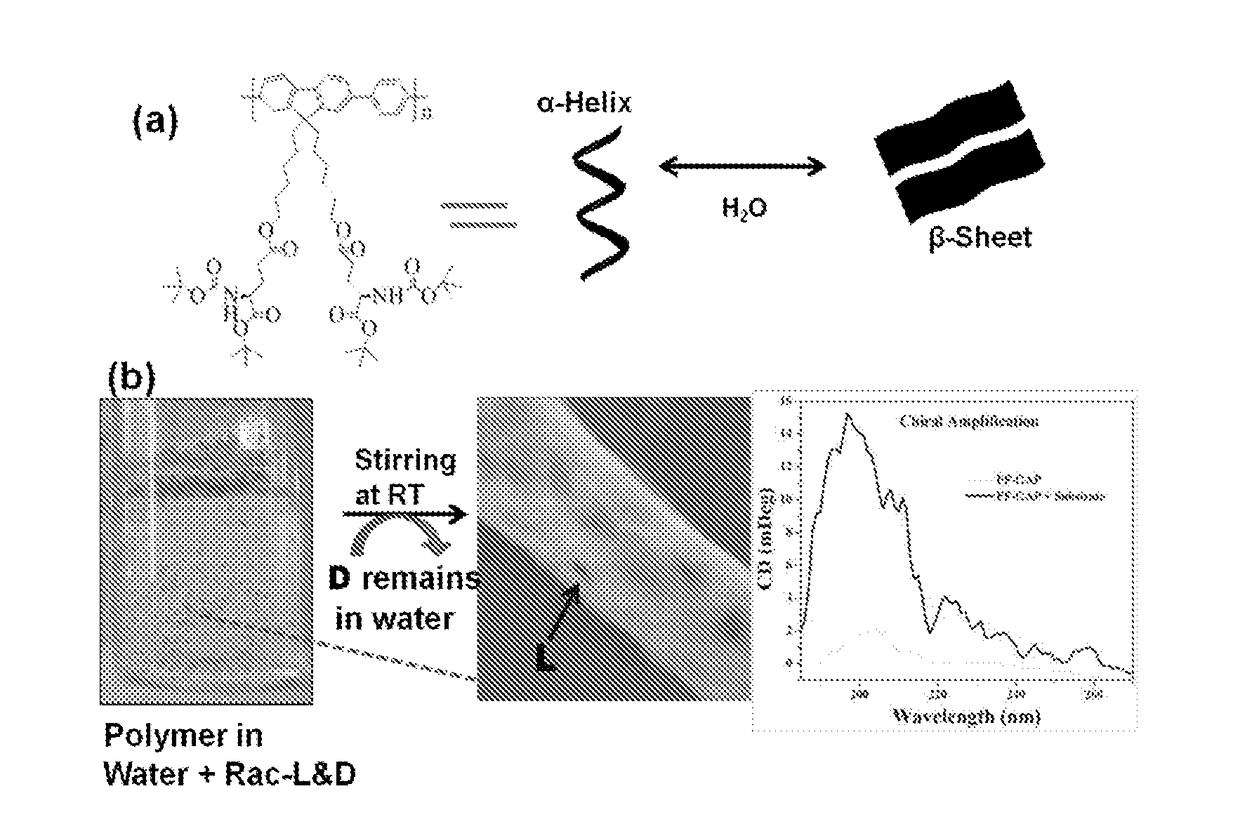
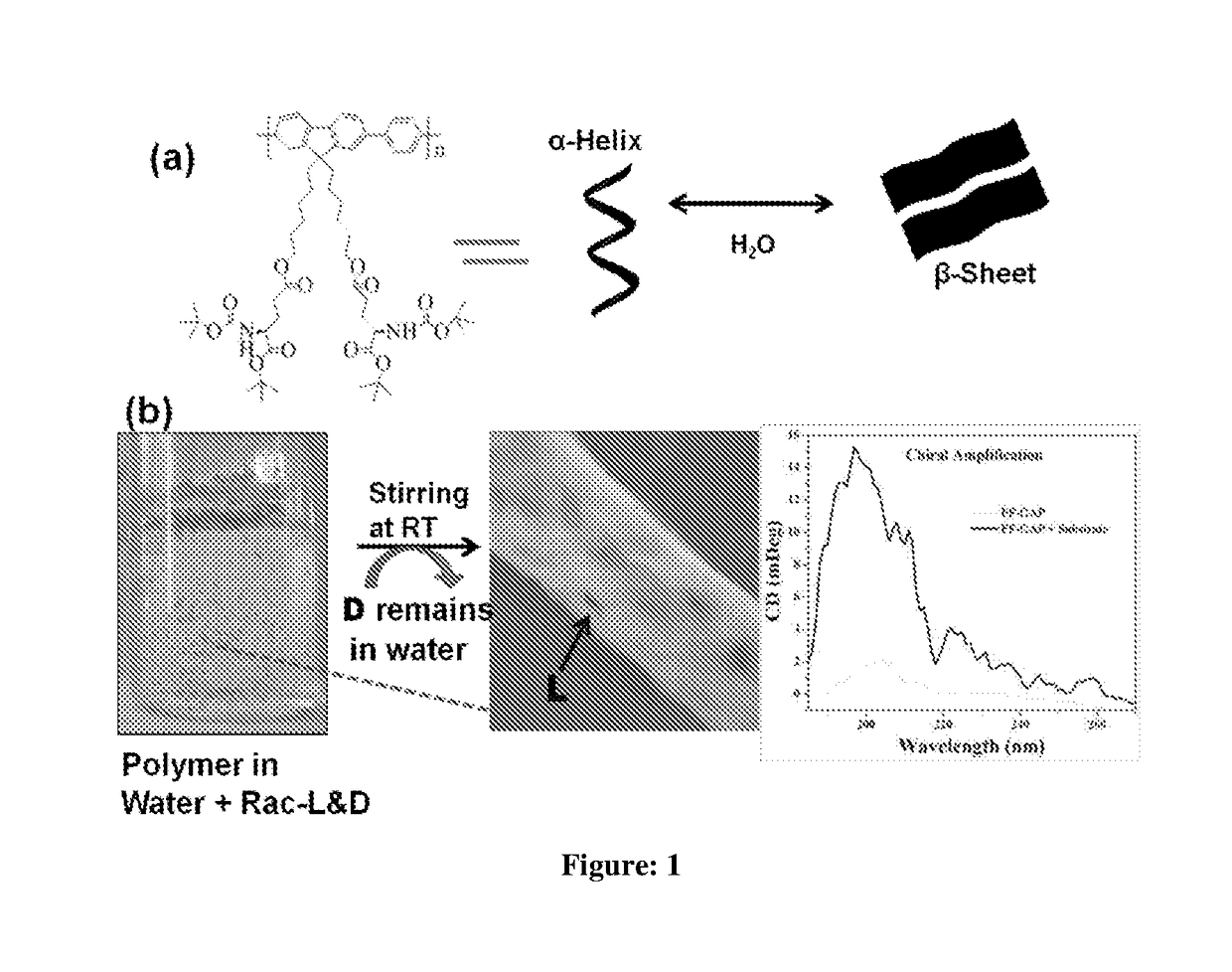
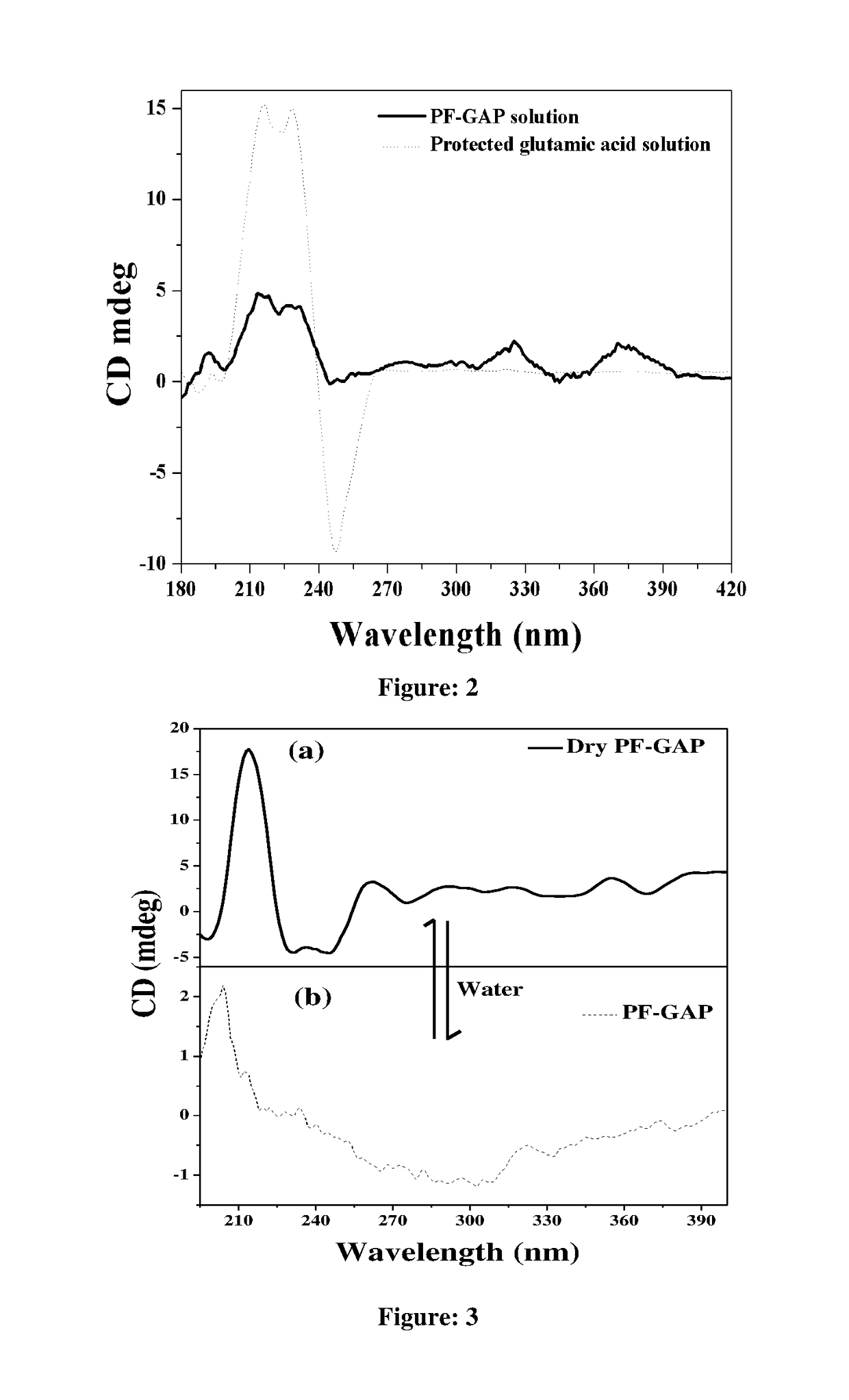
The present invention relates to a novel polyfluorene appended with protected glutamic acid of Formula (I) for heterogeneous enantioselective separation and sensing of amino acids, amino alcohol, hydroxyl acid, sugar, aromatic drug and ascorbic acid from racemic mixture in water and process for preparation thereof. The present invention further provides a process for separation of enantiomers and diastereomers into their individual isomers using a polyfluorene compounds of Formula (I).
Owner:COUNCIL OF SCI & IND RES
Synthetic method of gradient polyester
The invention discloses a synthetic method of gradient polyester. The invention belongs to the field of polymer synthetic materials. The invention aims to solve the technical problems of lack of research on chirality of a polymer chain and difficulty in synthesis and characterization in research on a chiral polymer in the prior art. The synthetic method of the gradient polyester comprises the following steps: under normal pressure and inert gas protection, in an organic solvent, racemic substituted lactone is used as a polymeric monomer, chiral phosphoric acid is used as a catalyst, and an alcohol compound is used as an initiator; and ring-opening resolution polymerization is realized by utilizing the difference of the reaction rates of two enantiomers in the polymeric monomer, so that the gradient polyester is obtained. According to the asymmetric resolution polymerization method for racemic substituted lactone, novel polyester with a gradient structure can be obtained, the types and application fields of polyester materials are expanded, and development of chiral polymer functional materials is promoted. The method is simple and convenient to operate, mild in condition and controllable in polymerization activity, and the generated polymer is free of metal residues.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Preparation method of bonded chiral stationary phase
InactiveCN101912772ASimple designEasy to splitOther chemical processesChemical reactionAtom-transfer radical-polymerization
The invention belongs to the field of chiral stationary phases, and particularly relates to a preparation method of a polysaccharide chiral stationary phase which is suitable for a high-efficiency liquid phase chromatography and uses silicon dioxide as a carrier. The preparation method specifically comprises the following steps of: (1) synthesizing a chiral polymer chain, namely using para-diacetone-D-galactose styrene as a monomer, and performing atom transfer free radical polymerization reaction to synthesize the chiral polymer chain poly-diacetone-D-galactose styrene; (2) preparing the silicon dioxide carrier, namely synthesizing silicon dioxide granules by adopting a Stober method, performing chloromethylation by using a chloromethylation silane coupling agent to obtain chloromethylation silicon dioxide granules, and reacting the chloromethylation silicon dioxide granules with sodium azide to obtain silicon dioxide azide granules; and (3) forming a covalent bond connection for bonding by performing click chemical reaction on the chiral polymer chain prepared in the step (1) and the chloromethylation silicon dioxide granules obtained in the step (2) so as to obtain the bonded chiral stationary phase. The preparation method can be used for designing chiral polymer chains with different molecular weight and silicon dioxide granules with different grain size so as to obtain chiral stationary phases with different performance.
Owner:SUZHOU UNIV
Method for preparing chiral carbon anion composite initiator
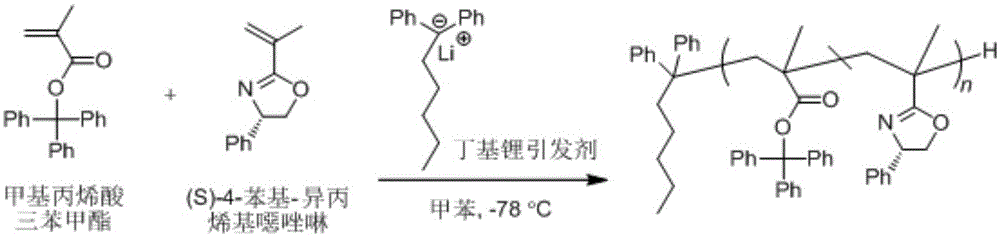
The invention discloses a method for preparing a chiral carbon anion composite initiator. The method comprises the following processes: (1) by taking chiral oxazole-substituted fluorine as a reagent, dissolving into tetrahydrofuran or methylbenzene; (2) adding pentane or hexane solution of lithium alkylide to the solution obtained in the step (1), and agitating at room temperature, so as to obtain a composite initiator solution; and (3) adding one or more monomer solutions to the composite initiator solution obtained in the step (2) to initiate anionic polymerization. By adopting the composite initiator applied to the method disclosed by the invention, chiral carbon can be introduced to the initial end of the polymer, so as to prepare the chiral polymer.
Owner:XIANGTAN UNIV
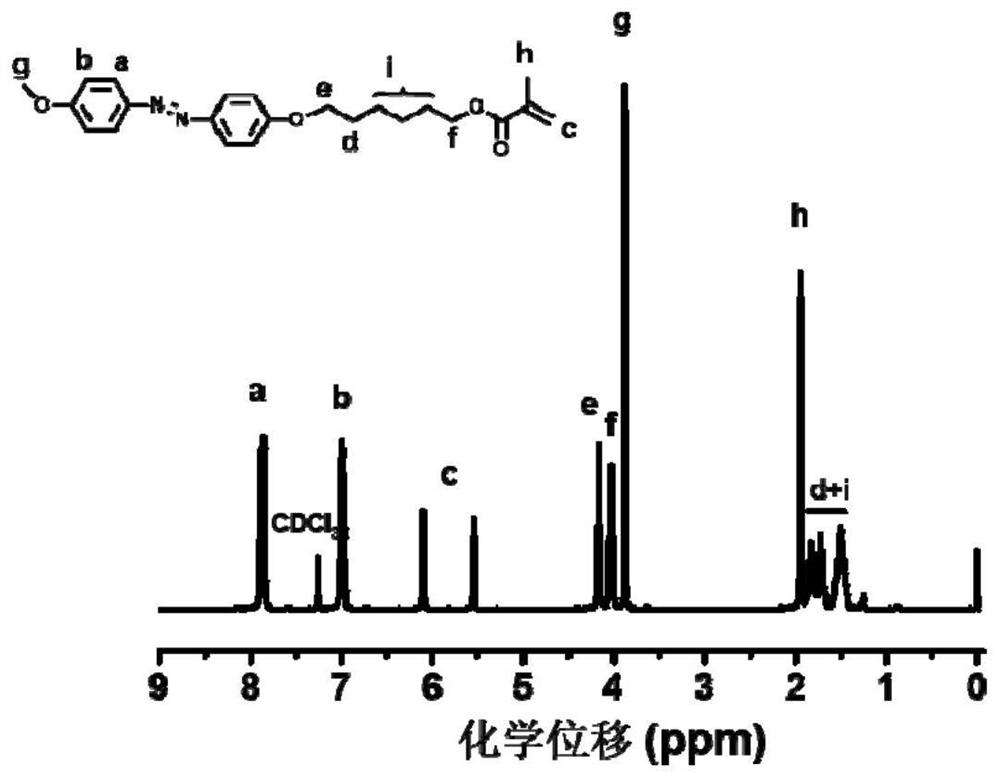
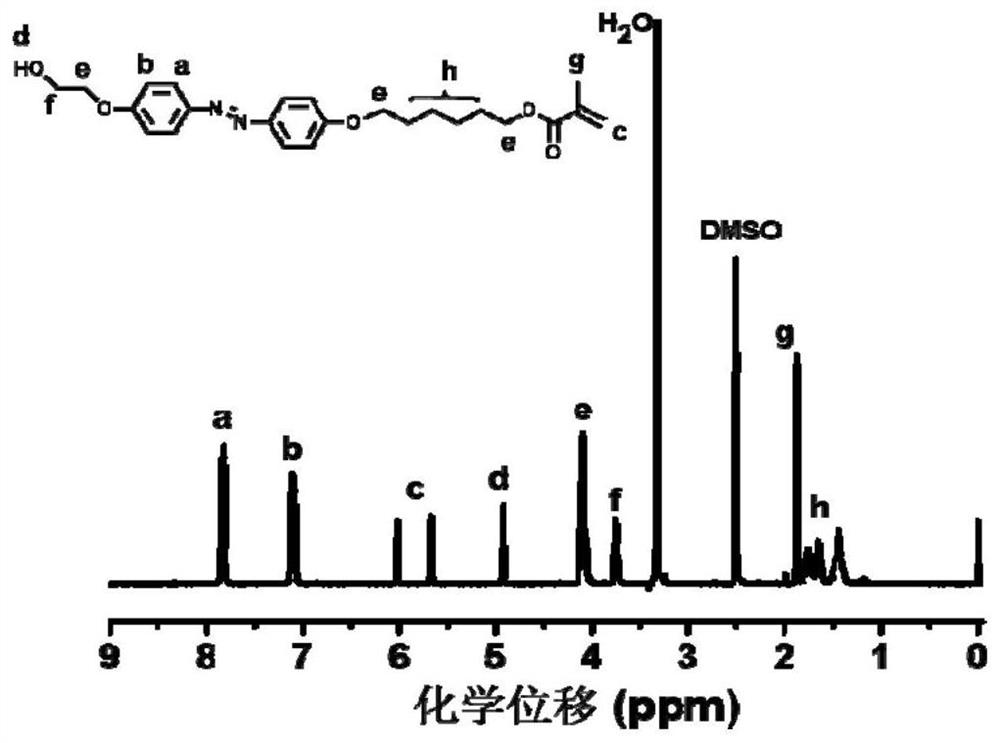
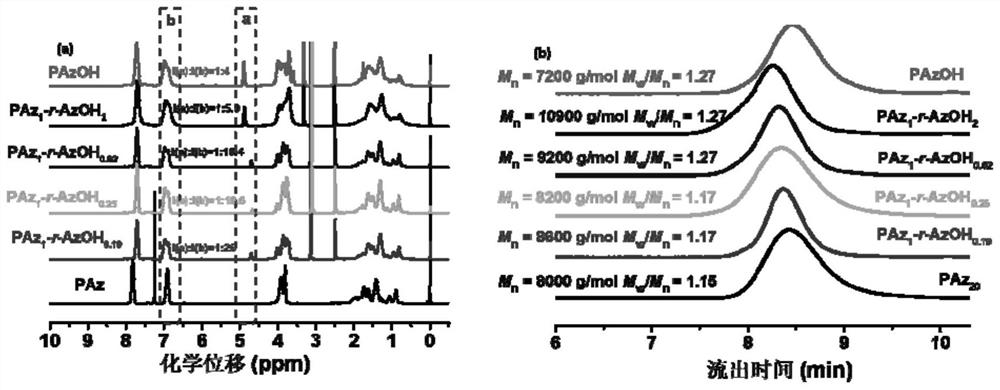
Chiral azobenzene polymer film as well as preparation method and application thereof
The invention discloses a chiral azobenzene polymer film as well as a preparation method and application thereof. A side chain-type azobenzene polymer is prepared into a film; and chiral reagent induction is carried out to obtain a chiral azobenzene polymer film. The method comprises the following steps: firstly, synthesizing an azobenzene random copolymer with hydroxyl at the tail end of a side chain through a series of organic synthesis reactions and RAFT polymerization, and carrying out detailed investigation on the molecular weight and the liquid crystal performance of the polymer by utilizing characterization means such as nuclear magnetism, GPC, DSC, POM and XRD; and preparing a polymer film in a spin-coating manner, and performing chiral induction on the polymer film by selecting chiral limonene steam to obtain an optically active polymer film. The chirality is transmitted from a chiral reagent to an achiral substance. The chiral solvent induction method provided by the invention is simple to operate, and avoids the complex synthesis steps of the chiral polymer and the use of expensive chiral reagents.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com