Method for constructing porous polymer on the basis of pure chiral molecules of 1, 1 '-bi-2-naphthol
A technology of porous polymers and chiral molecules, applied in the field of organic chemistry, can solve problems such as cumbersome methods and poor universal applicability of different ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] Preparation of Basic Framework Materials
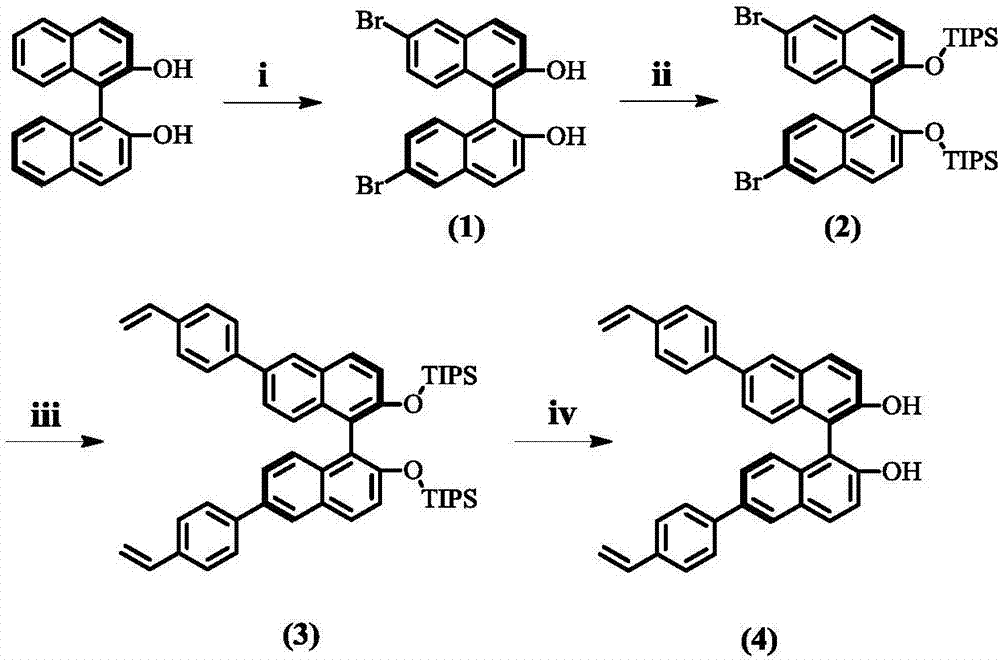
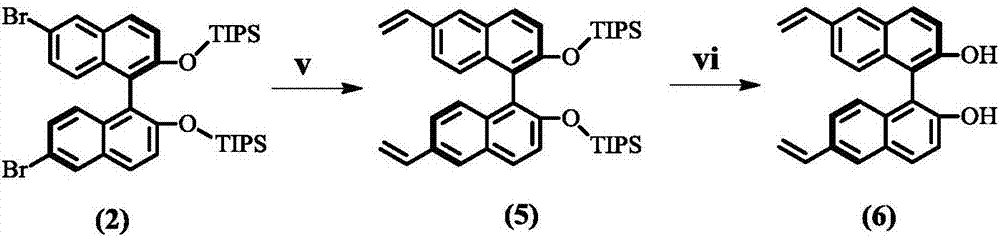
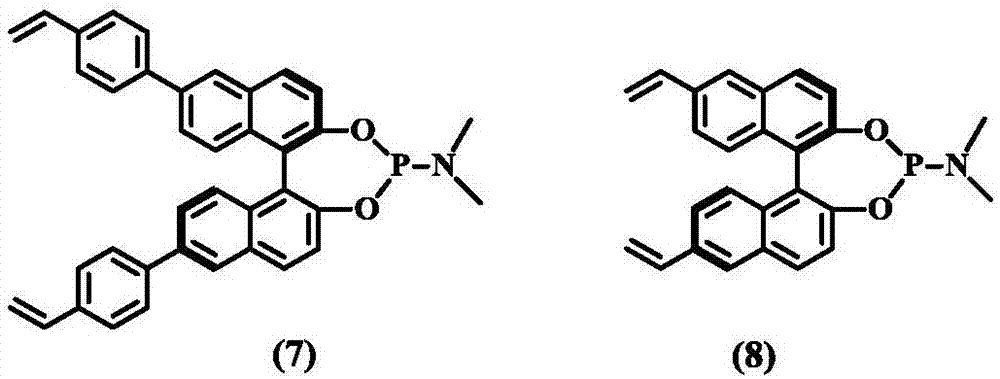
[0039] The basic skeleton material is a chiral ligand of vinyl-functionalized BINOL group, which is a chiral phosphoramidite ligand or chiral phosphite ligand obtained by reacting vinyl-functionalized BINOL with amine, alcohol or phenolic compound. body. Preparation routes such as figure 1 with figure 2 shown.
[0040] The synthesis steps of 6,6'-dibromo-[1,1'-binaphthyl]-2,2'-diol (compound 1) are as follows, 5.0 g of BINOL was dissolved in 100 mL of CH 2 Cl 2 , after the system temperature drops to -10°C, 2.4mL Br 2 with 20mL CH 2 Cl 2 Diluted and slowly added dropwise to the above solution. Add NaHSO 3 aqueous solution is quenched. CH for aqueous phase 2 Cl 2 Extracted with MgSO 4 Dry, rotary evaporate CH 2 Cl 2 The obtained white solid is compound 1.
[0041] The synthesis steps of 6,6'-dibromo-[1,1'-binaphthalene]-2,2'-diyldioxybis(triisopropylsilane) (compound 2) are as follows, 17.4mL of triisopropyl Ch...
Embodiment 1
[0050] 1 g of compound 7, 20 g of ethyl acetate, and 0.001 g of azobisisobutyronitrile were mixed and stirred, heated to 120° C. under stirring, reacted for 6 h, and the solvent was distilled to obtain a white polymer. of the sample 13 C MAS NMR and 31 P MAS NMR image as shown Image 6 with Figure 7 As shown, it can be seen that there is also a strong peak at 41.2ppm on the solid carbon NMR of the polymer, which indicates that the vinyl polymerization has successfully occurred. 31 The P MAS NMR chart shows that the chemical shift of the P NMR of the polymer is almost the same as that of the monomer, indicating that the valence state of P has not changed during the polymerization process. N 2 The adsorption results showed that the specific surface area of the obtained polymer was 490m 2 / g or so ( Figure 8 ), the pore size distribution is 0.5-150nm ( Figure 9 ).
Embodiment 2
[0052] 1 g of compound (8-20), 2 g of tetrahydrofuran, and 0.01 g of azobisisobutyronitrile were mixed and stirred, heated to 60° C. under stirring, reacted for 24 hours, and the solvent was distilled to obtain a white polymer. N 2 The adsorption results show that the specific surface area of the obtained polymer is 400-780g / m 2 About, between 1 ~ 100nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com