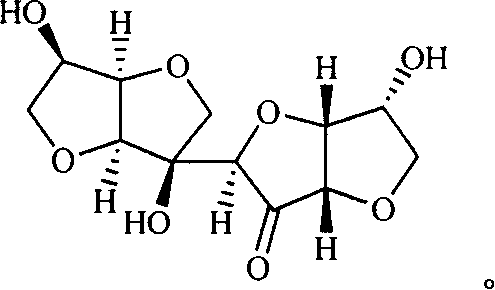
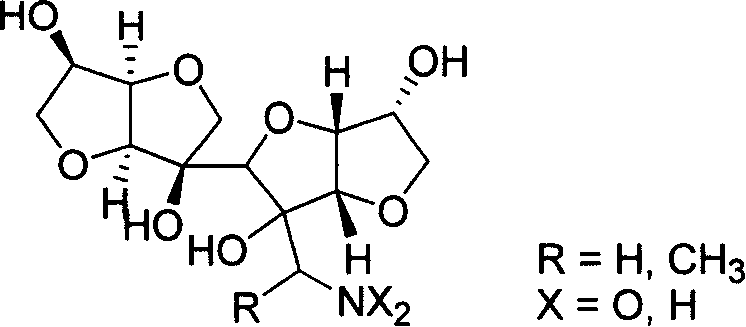
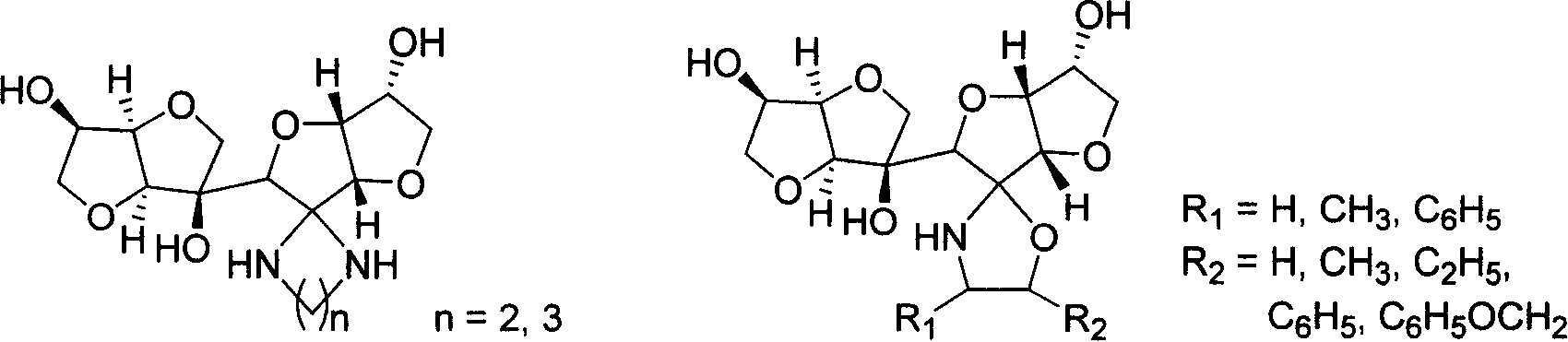
New typed sugar of C12 high carbon and ramification, preparation method and application
A technology of sugar derivatives and C12, which is applied in the field of sugar compounds and their preparation, can solve the problems of less high aldose and no high-carbon ketose synthesis, and achieve mild conditions, good market prospects, and high reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation C 12 high carbon ketose
[0032] 1,4:3,6-Bitrose (1.44g, 10mmol) was dissolved in methanol (10mL), a catalytic amount of sodium methoxide was added, and the mixture was refluxed for 6 hours. After the reaction, the reaction solution was concentrated, crystallized from absolute ethanol, and C was obtained. 12 High carbon ketose 1.15g, yield 80%. C 12 The data of the high-carbon ketose experiment are as follows.
[0033] C 12 h 16 o 8 , mp 190-191°C, [α] D 20 =+173.4(c 1.10, CH 3 OH); υ=3381,1771,1401,1128,1066,880,747cm -1 ; 1HNMR (400MHz, DMSO-d 6 ): δ4.87(dd, J=4.8, 6.8Hz, 1H), 4.39-4.41(m, 2H), 4.26(d, J=6.8Hz, 1H), 4.10-4.15(m, 2H), 3.92( s, 1H), 3.95(d, J=8.8Hz, 1H), 3.82(dd, J=4.8, 8.8Hz, 1H), 3.79(m, 1H), 3.54(dd, J=4.8, 8.8Hz, 1H ), 3.42(d, J=8.8Hz, 1H), 3.38(m, 1H);
[0034] 13 CNMR (100MHz, DMSO-d 6 ): δ212.8, 83.2, 81.4, 81.0, 80.3, 79.7, 78.4, 72.5, 72.3, 72.2, 71.8, 71.3.
Embodiment 2
[0035] Example 2 Preparation of Derivatives When X=O Shown in General Formula 1
[0036] The above prepared C 12 High carbon sugar (1.44g, 5mmol) was dissolved in ethanol (20mL), 0.5mL of nitromethane and 20mg of potassium fluoride were added, and reacted at 80°C for 5 hours. After the reaction was completed, the reaction solution was concentrated and recrystallized from methanol to obtain 1.66 g of the derivative represented by general formula 1 when X=O, with a yield of 95%. The experimental data are as follows.
[0037] C 13 h 19 NO 10 , mp 160-162°C, [α] D 20 =+92.9 (c=1.06, CH 3 OH), υ=3405, 2942, 2849, 1554, 1416, 1123, 1075, 859, 702cm -1 ; 1 HNMR (400MHz, D 2 O): δ4.95(d, J=13.6Hz, 1H), 4.85(d, J=13.6Hz, 1H), 4.60(t, J=4.0Hz, 1H), 4.59(d, J=5.4Hz, 1H), 4.48(d, J=4.0Hz, 1H), 4.31(m, 1H), 4.26(t, J=5.4Hz, 1H), 4.17(m, 1H), 3.91(s, 2H), 3.89( dd, J=6.4, 8.8Hz, 1H), 3.86(dd, J=6.8, 8.8Hz, 1H), 3.78(s, 1H), 3.50(t, J=8.8Hz, 1H), 3.45(t, J = 8.8Hz, 1H); 13 CNMR...
Embodiment 3
[0038] Example 3 Preparation of Derivatives When X=H Shown in General Formula 1
[0039] The product obtained in Example 2 (1.05 g, 3 mmol) was dissolved in methanol (120 mL), a catalyst 10% Pd / C (105 mg) was added, and hydrogenation was performed at 40° C. for 8 hours. After the reaction was completed, the reaction liquid was concentrated and crystallized from acetonitrile to obtain 852 mg of the derivative represented by general formula 1 when X=H, with a yield of 89%. The experimental data are as follows.
[0040] C 13 h 21 NO 8 , mp 76-78°C, [α] D 20 =+86.5 (c=0.32, CH 3 OH), υ=3367, 2946, 2872, 1131, 1082, 1042, 866cm -1 ; 1 HNMR (400MHz, D 2 O): δ4.66(d, J=4.4Hz, 1H), 4.65(t, J=4.4Hz, 1H), 4.32(m, 1H), 4.31(t, J=4.4Hz, 1H), 4.28( d, J=4.4Hz, 1H), 4.24(m, 1H), 3.95(s, 2H), 3.94(m, 1H), 3.87(s, 1H), 3.85(dd, J=6.4, 9.2Hz, 1H ), 3.58(t, J=8.4Hz, 1H), 3.52(dd, J=7.2, 9.2Hz, 1H), 3.28(s, 2H); 13 CNMR (100MHz, D 2 O): δ88.1, 85.9, 85.3, 80.9, 80.8, 80.4, 79.7, 74....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com