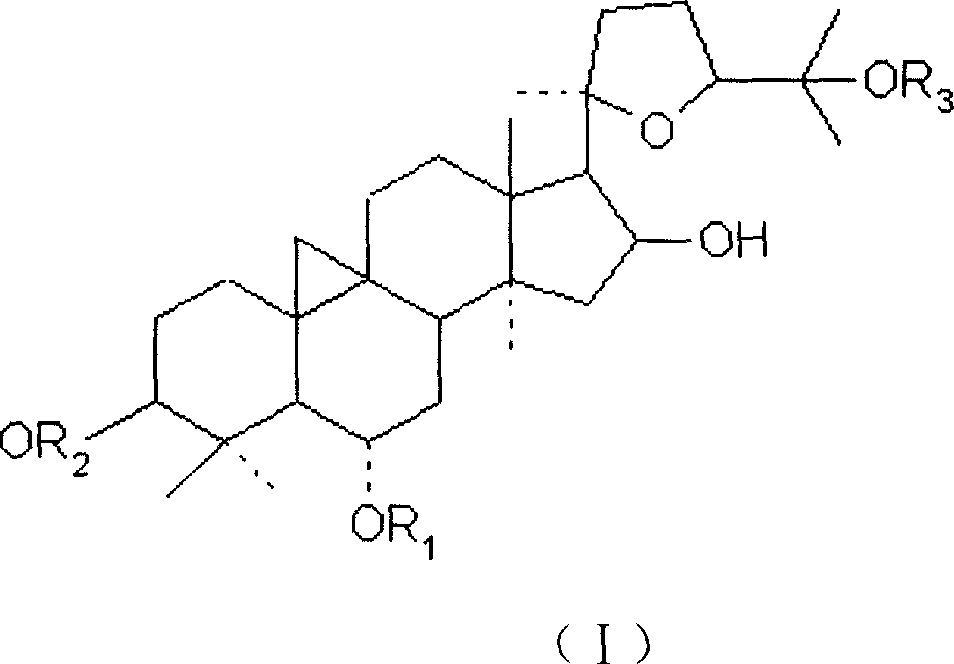
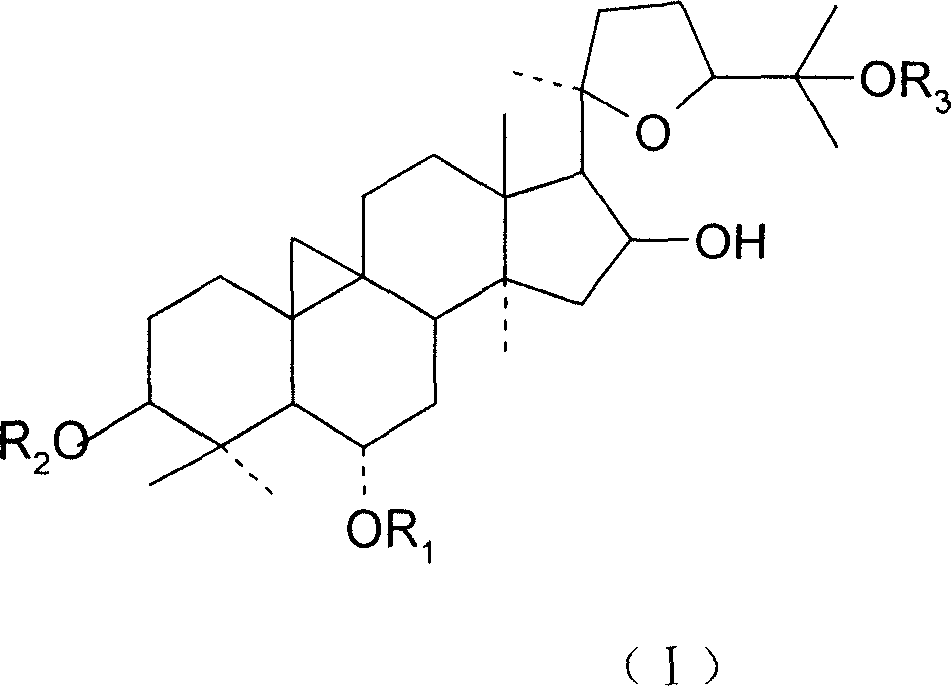
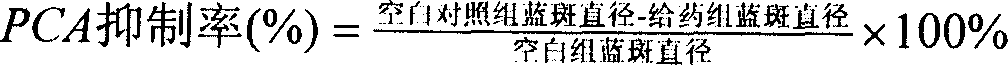Medicine for treating and preventing immune abnormalism disease
A technology for allergic diseases and autoimmunity, applied in the field of medicine, can solve problems such as incomplete elucidation of etiology and pathogenesis, exacerbation of glomerular immune inflammatory response, loss of skin pigmentation, etc., to achieve good anti-inflammatory and selective cell Effects of immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] [Example 1] Asthma test-ovalbumin aerosol inhalation method and cell count of alveolar lavage fluid
[0043] Get 40 guinea pigs of 200-250 g, half male and half male, and inject 0.2 ml of 4% albumin saline solution intramuscularly into the outer right hind leg, and simultaneously intraperitoneally inject 0.2 ml of 4% aluminum hydroxide gel for sensitization. From the second day of sensitization, the animals were randomly divided into 4 groups: control group, sodium chromoside group, cycloastragenol 20 mg / kg group and astragaloside IV group, and cycloastragel alcohol and astragaloside IV group were administered intragastrically Administration, injection administration of sodium chromylate group, totally 14 days, after the last medicine, animal is put in airtight glass bell jar, after being quiet, start air compressor, spray into with the constant pressure of 53kPa (400mmHg) 3.5% ovalbumin normal saline for 30s, observe the latency period of wheezing convulsions in guinea...
Embodiment 2
[0051] [Example 2] Type I Allergy 1-Passive Skin Allergy Test (PCA)
[0052] The rats were randomly divided into 4 groups according to body weight: blank control group, sodium chromylate group, cycloastragenol 20 mg / kg group and astragaloside IV 20 mg / kg group, with half male and half male. The cycloastragenol and astragaloside IV groups were administered by intragastric administration, and the sodium chromylate group was administered by injection. The test used preventive administration, and the administration was continued for 14 days before the antigen challenge.
[0053] Preparation of antiserum: Inject the above-mentioned trichosanthes aluminum hydroxide suspension into four paws, inject 0.1ml into each paw, a total of 0.4ml. After 10-15 days of maturity, about 10ml of eyeball bloodletting, centrifuge at 3000 rpm for 15min, take the upper serum The antiserum was obtained.
[0054] Passive skin sensitization: the backs of the rats in each test group were depilated, and th...
Embodiment 3
[0061] [Example three] type I allergy 2-mast cell degranulation test
[0062] 40 SD rats were randomly divided into 4 groups according to body weight: blank control group, cromolyn sodium, cycloastragenol 20mg / kg group and astragaloside IV 20mg / kg group, 10 rats in each group, half male and half female, administered once / d, continuous administration for 14 days. 0.1-0.2 ml of anti-ovalbumin serum diluted 1:5 was subcutaneously injected into the head of the rat, and 48 hours later, 1 mg of ovalbumin-Evans blue solution was injected into the tail vein for challenge. The animals were killed 30 minutes after the challenge, the skin of the head was peeled off, the skull was removed, treated with 95% ethanol for 1 hour, and then put into anhydrous methanol overnight. After the mast cells were treated with 0.18% neutral red, they were rinsed with running water, one side of the skull was fixed on a small wooden board with a pin, the periosteum was carefully peeled off with tweezers,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com