Preparation method of bonded chiral stationary phase
A chiral stationary phase, bonding type technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of low sample loading, low grafting rate, troublesome operation, etc., to facilitate large-scale production. , the reaction operation is simple, the effect of less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
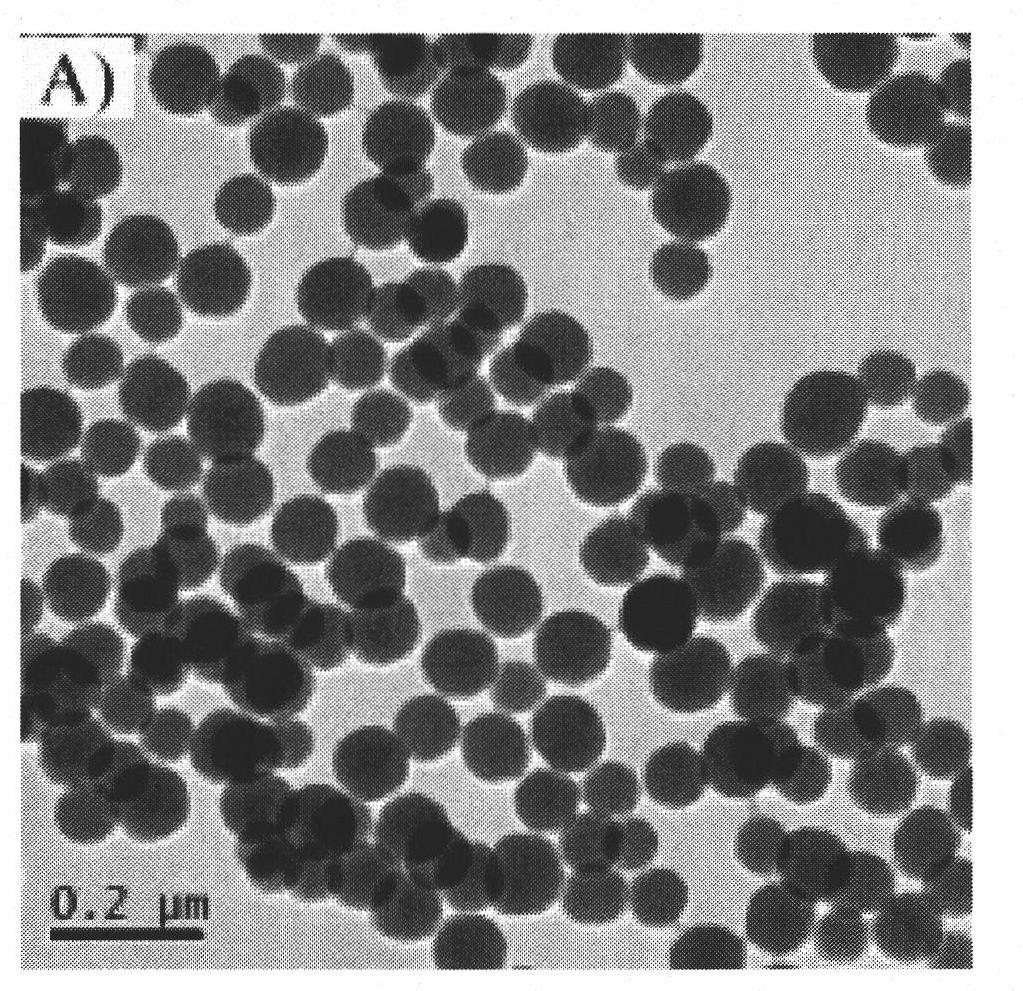
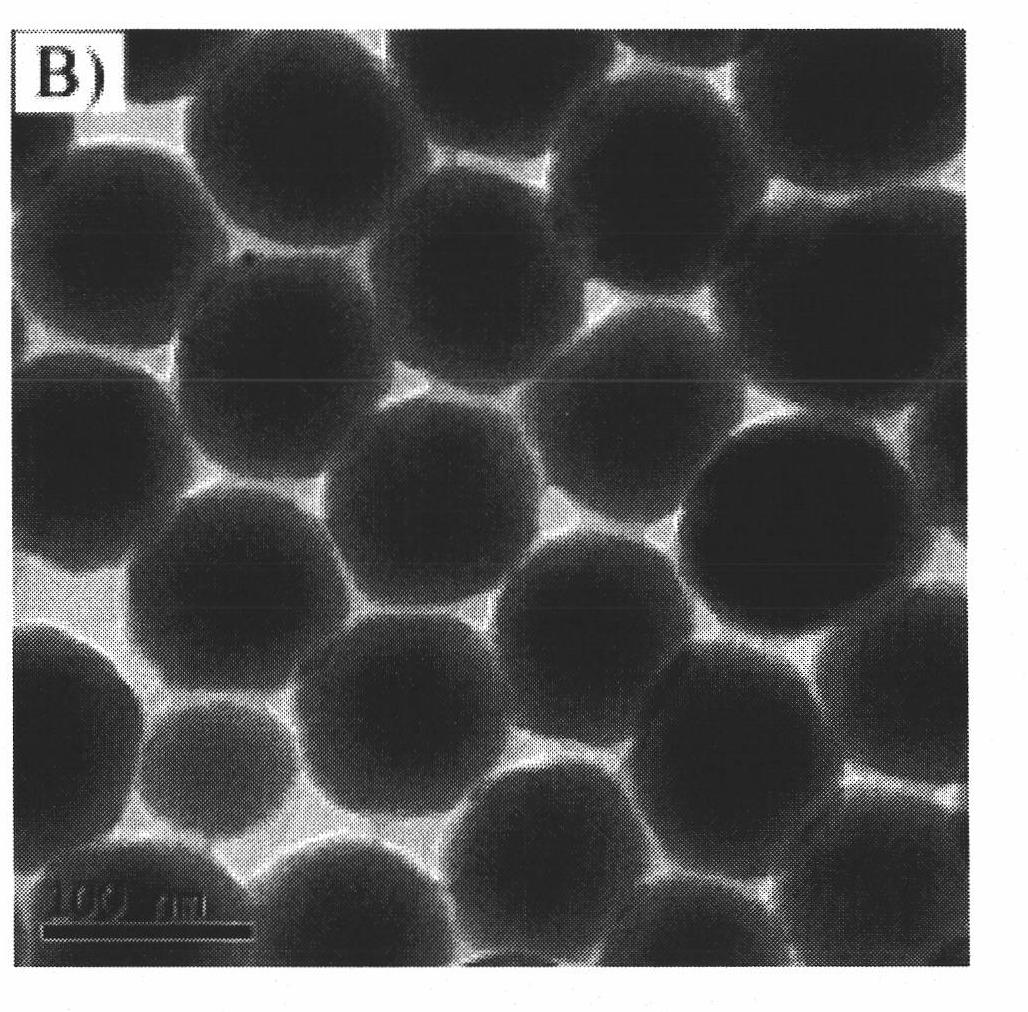
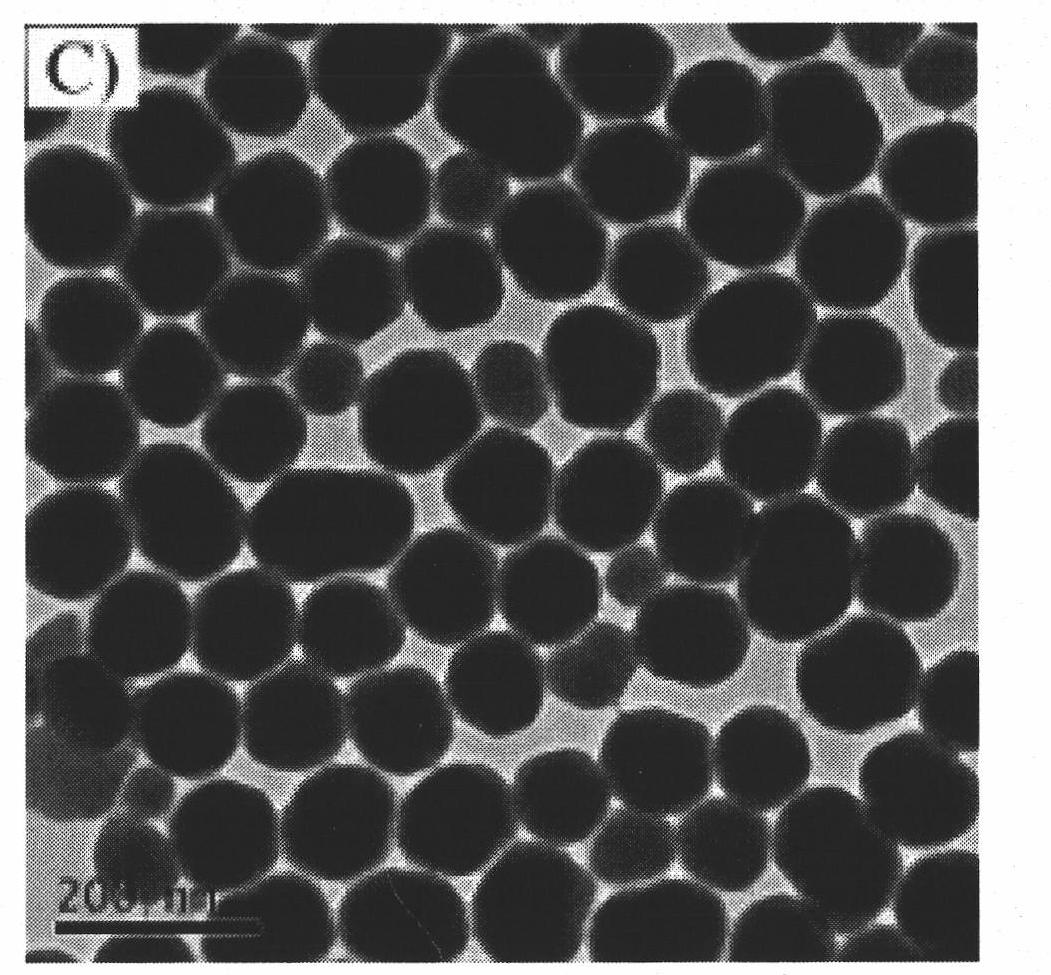
Image
Examples
Embodiment 1
[0037] Embodiment one: the synthesis of diacetone-D-galactose styrene (VBPG)
[0038] Drop 2.36g of p-chloromethylstyrene into a mixed aqueous solution (30mL) of 12g NaOH and 0.3g tetrabutylammonium bromide, and stir at room temperature for several minutes; dissolve 2.89g of diacetone-D-galactose (PG) In 30 mL of tetrahydrofuran, slowly drop the above mixed aqueous solution, stir at room temperature, and react for 24 h. After the reaction was completed, the reaction solution was washed with chloroform and distilled water until the solution was neutral, and the crude product was a yellow liquid in chloroform, and then purified with a chromatographic column (silica gel column, sherwood oil / ethyl acetate=8 / 1 (volume ratio)). The final product, VBPG, was a white snowflake solid with a yield of 46%.
Embodiment 2
[0039] Example two: α-butynyl bromoisobutyrate (BEiB)
[0040] At 0°C, 103g, 0.45mol bromoisobutyryl bromide in anhydrous ethyl acetate solution 200mL, was added dropwise to 150mL ethyl acetate solution containing 37.1g (0.53mol) n-butynol and 43g (0.53mol) pyridine , Stir the reaction for 2 h, and then react overnight at room temperature. After the reaction, wash with water, sodium bicarbonate hydrochloric acid solution and saturated brine twice respectively, dry and distill to obtain 70.0 g of butynyl α-bromoisobutyrate.
Embodiment 3
[0041] Example 3: Synthesis of functional P(VBPG) chiral chains using BEiB as an initiator
[0042] According to the ratio n(VBPG):n(BEiB):n(CuBr):n(PMDETA)=75:1:1:3. Add CuBr, PMDETA, BEiB, VBPG and toluene (5 mL) in sequence to a 10 mL ampoule, and seal the tube after argon gas for 10 minutes. The sealed ampoule was placed in an oil bath at a constant temperature (75° C.) for a predetermined time to react (6-18 days). After the reaction is over, take out the sealed tube, immediately cool it with cold water, open the sealed tube, precipitate in an appropriate amount of petroleum ether, and dry to obtain the chiral polymer.
[0043] Using bifunctional initiator (BEiB) and VBPG as monomers, the data of chiral polymer chains synthesized at different polymerization times are shown in Table 1. As can be seen from the data in the table, the chiral polymer molecular weight obtained by solution polymerization increases linearly with the polymerization time, and the molecular weight...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com