Applications of chiral polymer catalyst in asymmetric reaction
A polymer and catalyst technology, applied in the field of preparation of heterogeneous catalysts, can solve the problems of difficult catalyst recovery, low activity of heterogeneous asymmetric reaction catalysts, etc., and achieve high stability, shortened reaction time, and optimized reaction activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
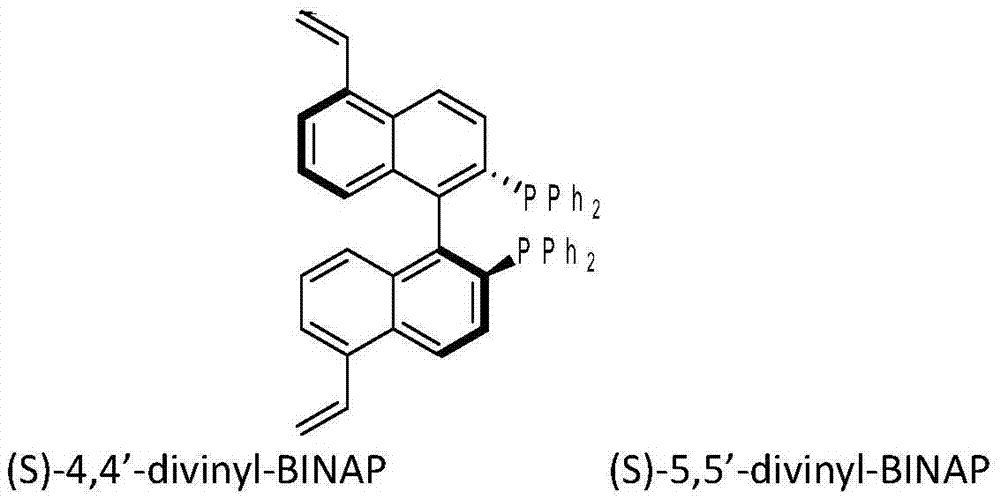
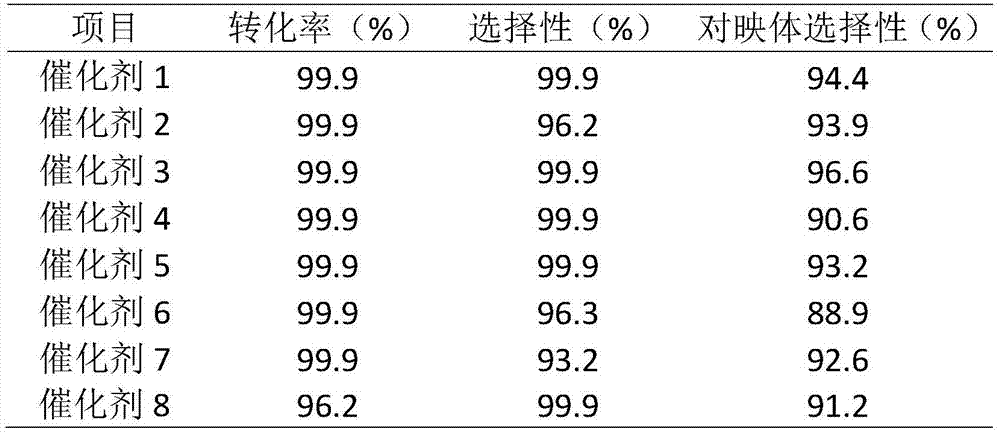
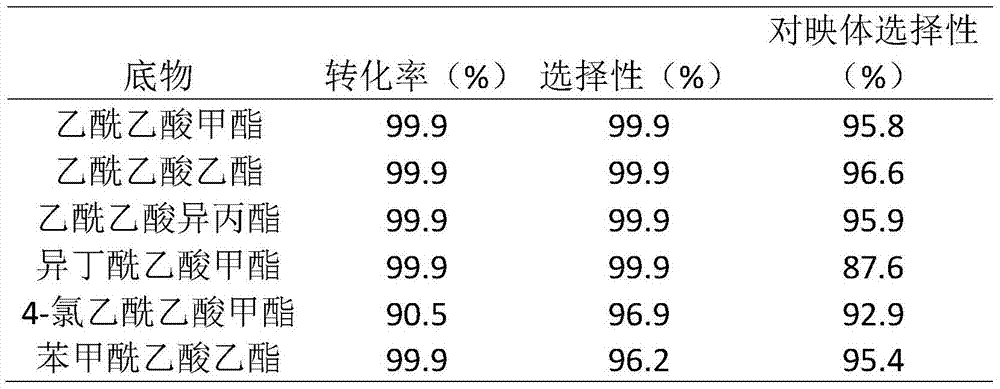
[0037] Dissolve 5.0g of (S)-5,5'-divinyl-BINAP ligand in 50mL tetrahydrofuran solvent at 25°C under an inert gas atmosphere, add 50.0g of divinylbenzene monomer, stir well, then slowly add 0.5g free radical initiator azobisisobutyronitrile, stirred for 0.5h, then transferred the solution to a polytetrafluoro-lined kettle, and solvothermally polymerized at 100°C for 24h. After the reaction, the reaction product was vacuum-dried at 65° C. for 10 h to obtain a porous chiral polymer. Take 0.044g [RuCl 2 (benzene)] 2 Dissolve in 8mL of N,N-dimethylformamide solvent, add 2.4g of polymer material, and stir at 100°C for 3h under an inert gas atmosphere. Then vacuum dry at 75°C for 10 h. The heterogeneous catalyst 1 for the asymmetric reaction of β-keto esters was obtained.
Embodiment 3
[0039] In Example 3, except that 5.0g (S)-5,5'-divinyl-BINAP ligand was replaced by 5.0g (S)-4,4'-divinyl-BINAP ligand, the rest of the operations were the same as in Example 2 Similarly, catalyst 2 was prepared.
Embodiment 4
[0041] In Example 4, except that 5.0g (S)-5,5'-divinyl-BINAP ligand was replaced with 12.5g (S)-5,5'-divinyl-BINAP, the rest of the operations were the same as in Example 2, Catalyst 3 was prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com