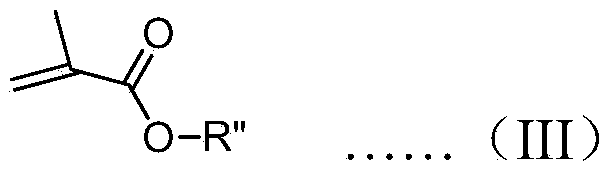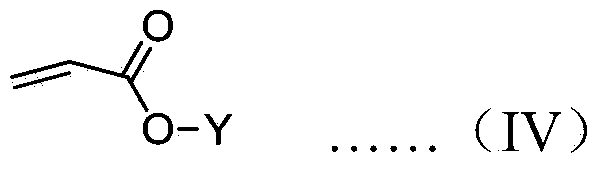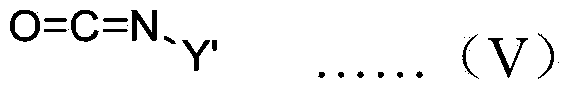Method for preparing chiral carbon anion composite initiator
An initiator and anion technology, which is applied in the field of preparation of chiral carbanion combined initiators, can solve the problems that optically active polymers cannot be obtained, the types are few, and the polymerization is greatly affected by the solvent, etc. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of a chiral carbanion combination initiator, comprising the steps of:
[0046] Dissolve 10 mmol of (S)-type (4-isopropyloxazole) 1-substituted fluorene in toluene, add an equivalent amount of n-butyllithium pentane solution, and stir at room temperature to obtain a combined initiator solution.
[0047] Drop this initiator solution into a toluene solution containing 20 times the equivalent of ethyl methacrylate at -78°C under the protection of nitrogen, stir for 2 hours, drop into methanol to terminate the polymerization, and then drop the mixed solution into a large amount of methanol to obtain a polymer , the measured specific rotation at 365nm is +2.8°.
Embodiment 2
[0049] A preparation method of a chiral carbanion combination initiator, comprising the steps of:
[0050] Dissolve 10 mmol of (S) type (4-tert-butyloxazole) 2-substituted fluorene in toluene, add an equivalent amount of n-butyllithium pentane solution, and stir at room temperature to obtain a combined initiator solution.
[0051] Drop this initiator solution into a toluene solution containing 50 times the equivalent of butyl isocyanate at -78°C under the protection of nitrogen, stir for 2 hours, drop into methanol to terminate the polymerization, and then drop the mixed solution into a large amount of methanol to obtain a polymer , the measured specific rotation at 436nm is -15.2°.
Embodiment 3
[0053] A preparation method of a chiral carbanion combination initiator, comprising the steps of:
[0054] Dissolve 10 mmol of (R) type (4-benzyloxazole) 1-substituted fluorene in tetrahydrofuran, add an equivalent amount of sec-butyllithium hexane solution, and stir at room temperature to obtain a combined initiator solution.
[0055] Drop the initiator solution into the tetrahydrofuran solution containing 50 times the equivalent of diphenylmethyl acrylate under the protection of nitrogen at -78°C, stir for 2 hours, drop methanol to terminate the polymerization, and then drop the mixed solution into a large amount of methanol to obtain polymerization The measured specific rotation at 365nm is +17.3°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com