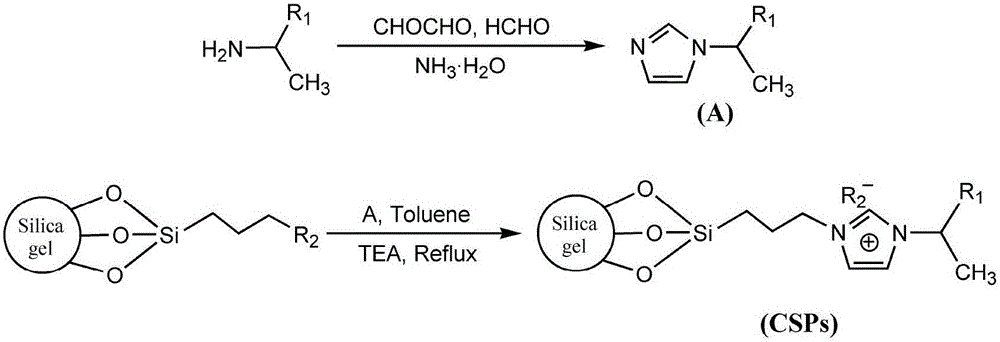
Imidazole ionic liquid chiral stationary phase and preparation method and application
A chiral stationary phase, ionic liquid technology, applied in separation methods, chemical instruments and methods, solid adsorbent liquid separation, etc. problems, to achieve the effect of good chiral separation, easy availability of raw materials, novel and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of chiral imidazole
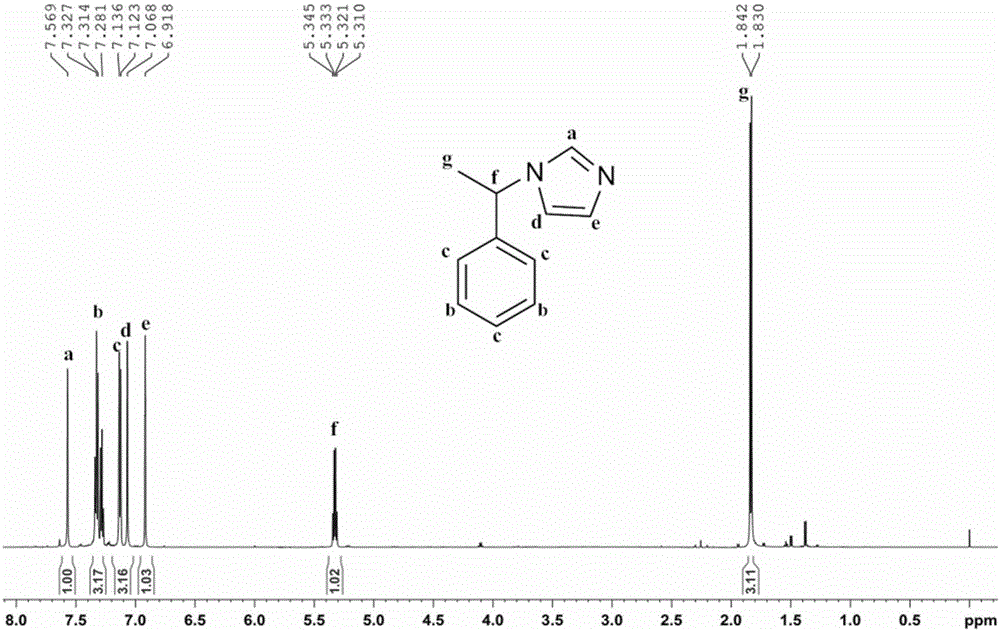
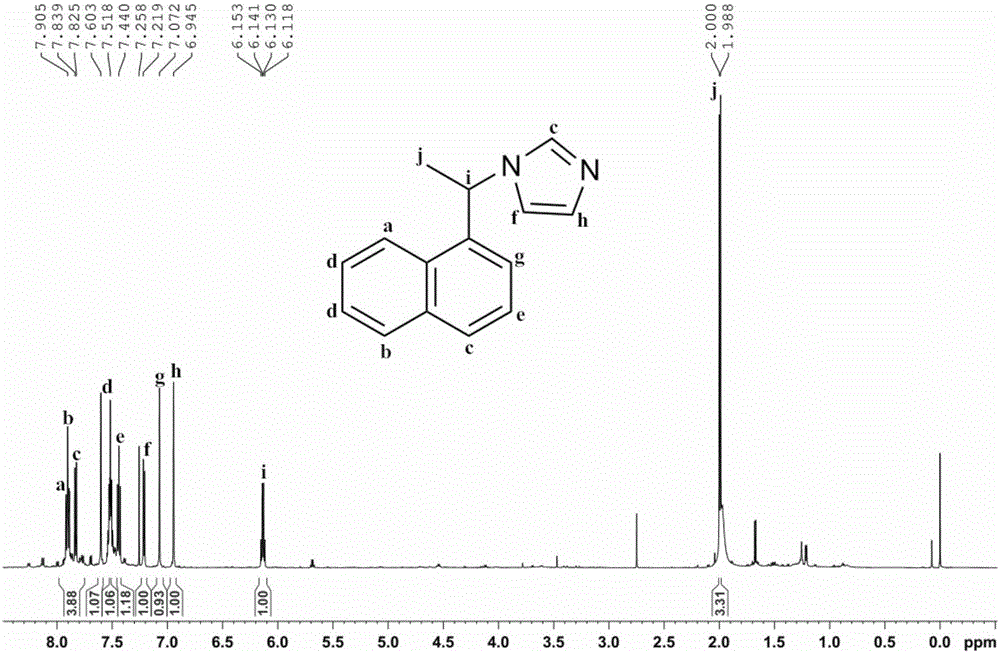
[0039] Mix (S)-1-phenylethylamine (14.69g, >99%, 0.12mol) and ammonia water (8.174g, 25-28%, 0.12mol), and its mixture is called solution A; Glyoxal Aqueous solution (17.41g, >40%, 0.12mol) and formaldehyde aqueous solution (9.74g, >37%, 0.12mol) are mixed uniformly, and its mixture is called solution B. Add solution A and solution B dropwise from two dropping funnels into a 150mL three-necked flask at the same time, keep the reaction slightly boiling under stirring, dropwise is completed in about 10 minutes, continue to stir and reflux for 3 hours and then stop. After the reaction is completed, distill the water under reduced pressure with a water pump, then use an oil pump to distill the product, and collect the fraction at 150-160°C (5-10mmHg). The product chiral N-(α-methylbenzyl)imidazole is a light yellow liquid (17.55g, yield 85%), figure 2 The structure of the product and its 1 H NMR spectrogram, it can b...
Embodiment 2
[0041] Example 2: Preparation of imidazole ionic liquid chiral stationary phase
[0042] Put 13g of blank silica gel into a 250mL three-necked flask, and add 130mL of 10% hydrochloric acid aqueous solution, stir and heat to reflux, suction filter after 8-10hr, wash with distilled water until neutral, then put it in an oven, 110°C Dry for 12 hours to obtain activated silica gel.
[0043] Weigh 12 g of activated silica gel and place it in a 150 mL three-necked flask, add 95 mL of anhydrous toluene, 10.5 mL of 3-chloropropyltrimethoxysilane, and 100 μL of triethylamine as a catalyst through a dropping funnel. Under the protection of nitrogen, the reaction was refluxed for 24 hours. After the reaction was completed, filter, bonded silica gel was extracted with acetone for 24 hours, and after drying, it was capped with 6 mL trimethylchlorosilane, and the reaction time was about 6 hours. After capping and filtering, the bonded silica gel was extracted with acetone for 24 hours, an...
Embodiment 3
[0048] Embodiment 3: the preparation of chiral chromatographic column
[0049] Using the homogenization method, the resulting chiral stationary phase was packed into a 4.6mm×250mm HPLC column: Disperse 3.5g of the target stationary phase filler in 30mL of n-hexane / isopropanol (90 / 10, v / v) A homogenate was made, and 40 mL of n-hexane was used as a displacement liquid, and filled into a stainless steel column under a pressure of 5800 psi. Utilize high-performance liquid chromatography, use biphenyl as the detection substance to measure retention time and column efficiency, the mobile phase is methanol / water=90 / 10 (v / v), isocratic elution, the detection wavelength is DAD 254nm, and the flow rate is 1.0 mL / min. The prepared chromatographic column was tested for column efficiency, and the results are shown in Table 2.
[0050] Table 2 Column efficiency measurement results
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com