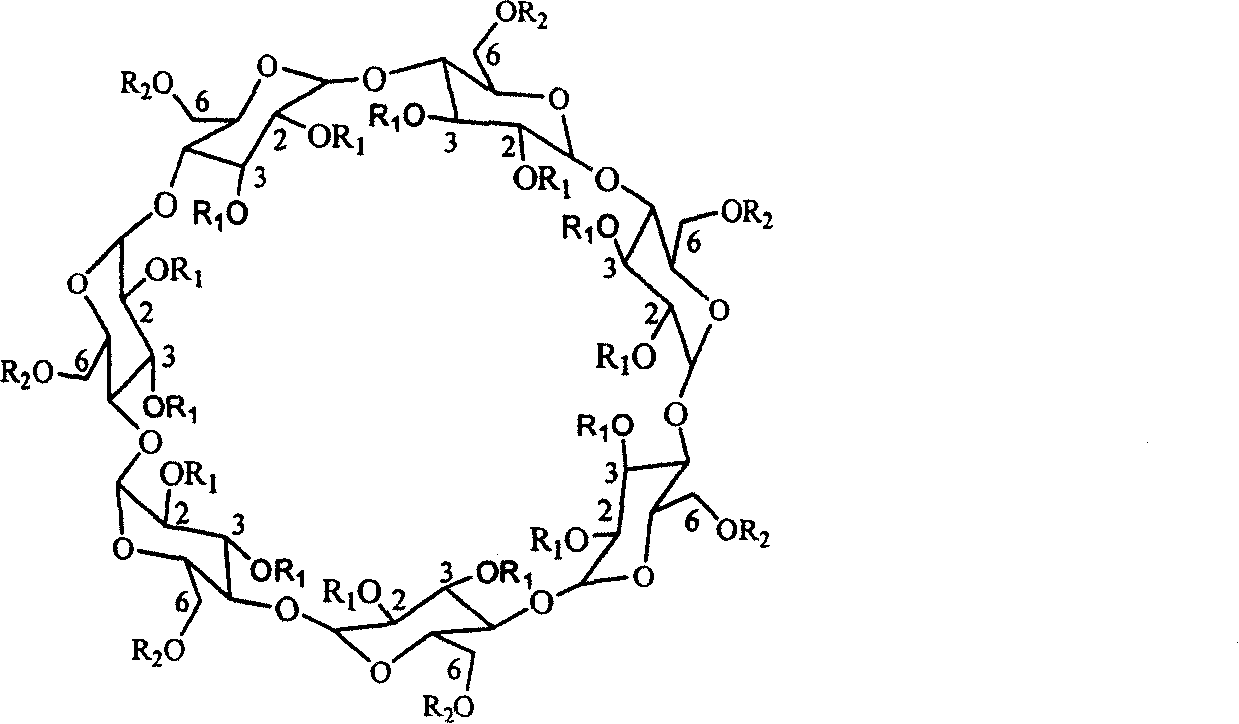
Beta-cyclodextrin derivative capillary gas chromatography chiral fixed phase and preparing method thereof
A chiral stationary phase, gas chromatography technology, used in chemical instruments and methods, other chemical processes, etc., to achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
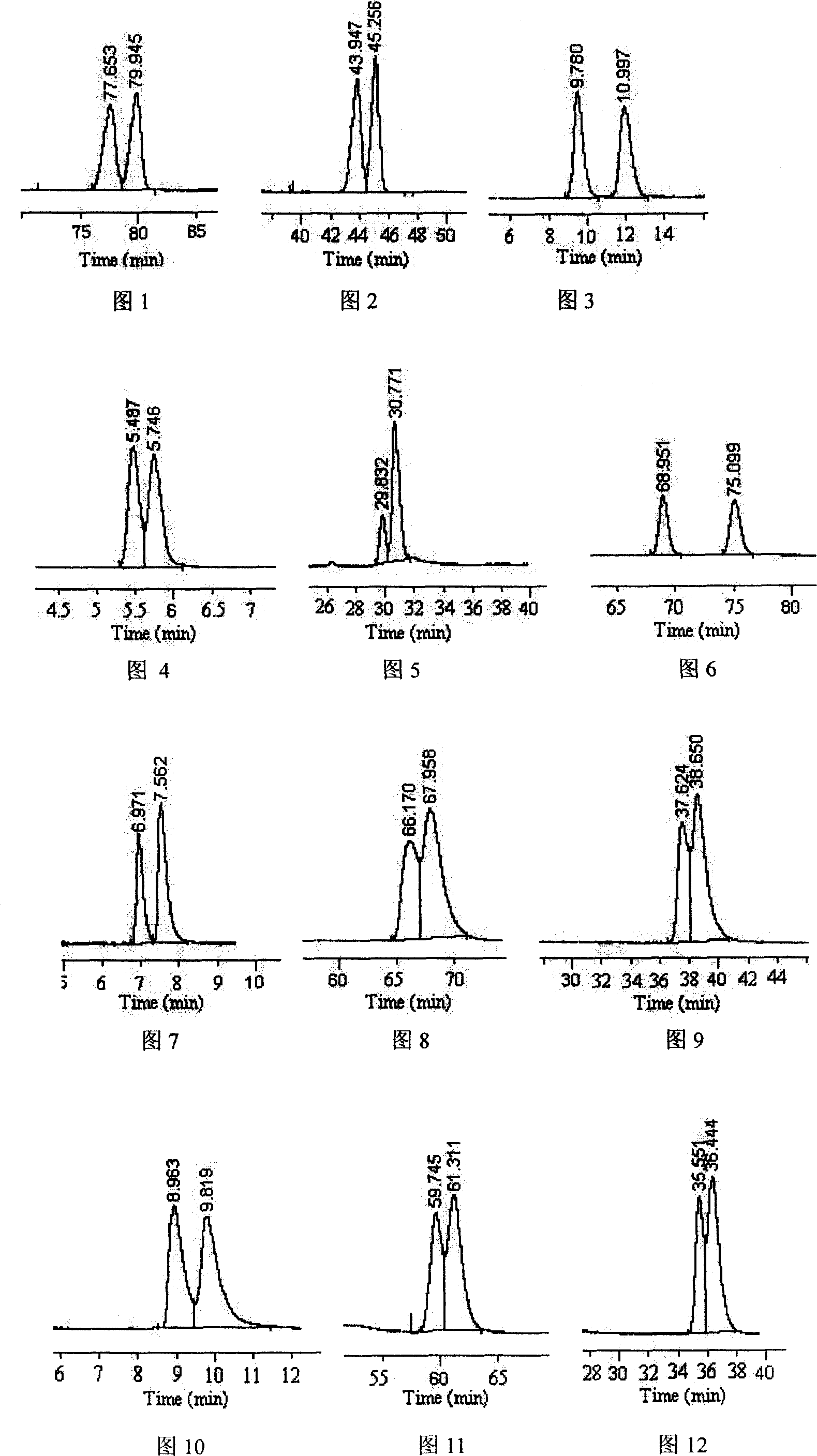
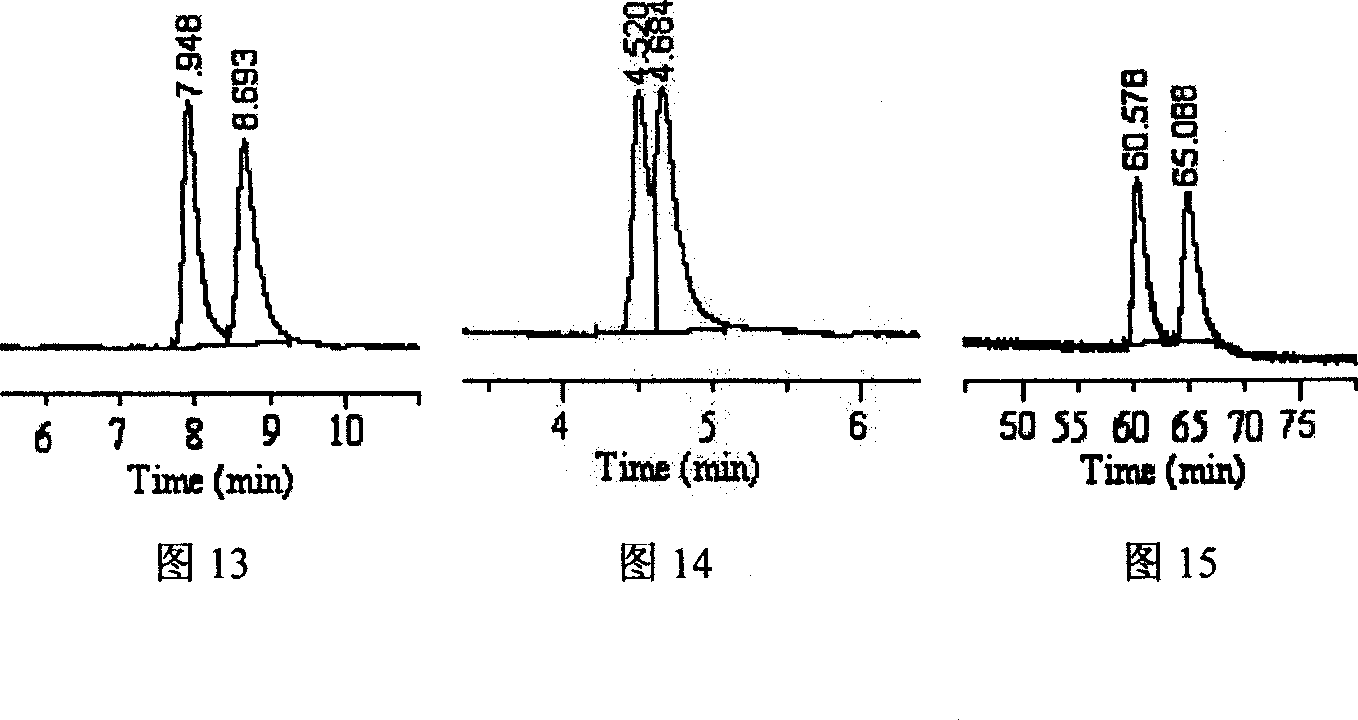
Image
Examples
example 1
[0056] Example 1: Preparation of 6-O-tert-butyldimethylsilyl-β-cyclodextrin (6-O-TBS-β-cyclodextrin)
[0057]Dissolve 3.36g (2.96mmol) of β-cyclodextrin in 45 mL of dry pyridine, add dropwise 3.44g (22.78mmol) of tert-butyldimethylsilyl chloride in 30mL of dry pyridine solution at 0°C, and stir at 0°C for reaction 3 h, after stirring and reacting at room temperature overnight, pour into an equal amount of ice water, stir, and a white precipitate appears after standing, collect the white precipitate, wash twice with 200 mL of water, filter and dry to obtain the product.
[0058] 1 H NMR (300MHz, CDCl 3 ): 0.66ppm (6H, Si-C H 3 ), 0.87(9H, C(C H 3 ) 3 ), 3.3~4.0 (H on CD ring, 6H), 4.89~4.95 (1H, CD anomeric H), 5.2~5.31 (1H, O H ), 6.6~6.8 (1H, OH)
example 2
[0059] Example 2: Preparation of 2,3-di-O-allyl-6-O-tert-butyldimethylsilane-β-cyclodextrin
[0060] Add 2g of 50% sodium hydride into a three-necked flask, add 90mL of dry dimethylformamide (DMF), cool to 0°C, add dropwise 3g of 6-O-tert-butyldimethylsilyl-β-cyclodextrin (6- (O-TBS-β-cyclodextrin) in 60mL of DMF solution, after the dropwise addition, react at room temperature for 2 hours, cool to 0°C in an ice bath, add 8mL bromide propene dropwise, react under ice bath for 1 hour, and react at room temperature for 24 hours. After the reaction was completed, excess sodium hydride was removed with methanol, DMF was distilled off under reduced pressure, extracted with 50 mL of chloroform × 3, the chloroform layers were combined, washed twice with 50 mL of saturated sodium chloride water and three times with 50 mL of water, dried and reduced Concentrate under pressure to obtain the product.
[0061] IR (cm -1 ): 3080 (C=CH 2 ), 2940, 2900, 2860 (CH 2 , CH 3 ), 1643 (C=C), 1...
example 3
[0062] Example 3: Preparation of 2,3-di-O-allyl-β-cyclodextrin
[0063] 5mL tetrabutylammonium fluoride (1mol L -1 THF solution) was added to 3.1g of 2,3-di-O-allyl-6-O-tert-butyldimethylsilane-β-cyclodextrin in tetrahydrofuran (THF) 10mL solution, reflux reaction for 70min, and reduced pressure After concentration, 20 mL of chloroform was added to dissolve the product, and the chloroform layer was washed three times with 20 mL of saturated sodium chloride water and twice with 20 mL of water, dried over anhydrous sodium sulfate, and concentrated to obtain the product.
[0064] IR (cm -1 ): 3445(O-H), 3079(C=CH 2 ), 2940, 2924, 2850 (CH 3 , CH 2 ), 1644 (C=C), 1458, 1415, 1350 (CH 3 , CH 2 ), 1160, 1090, 1040 (C-O-C), 923 (CH=CH 2 ) 1 H NMR (300MHz, CDCl 3 ): 0.88 (C H 3 CH 2 ), 1.25 (CH 3 C H 2 ), 3.3-4.4 (CD ( H ), OC H 2 ), 5.0-5.28 (CD ( H ), CH=C H 2 ), 5.9 (C H =CH 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com