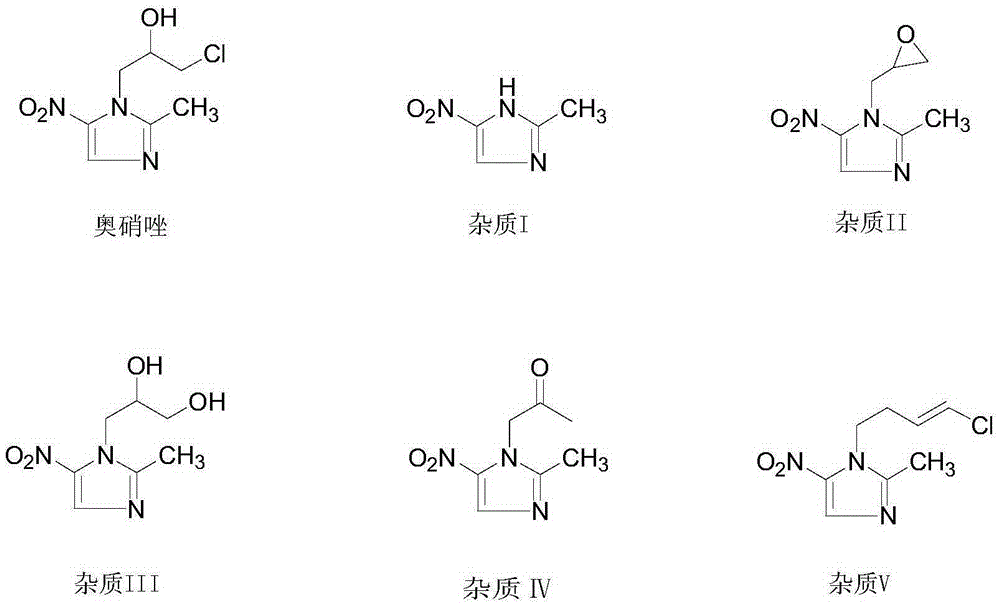
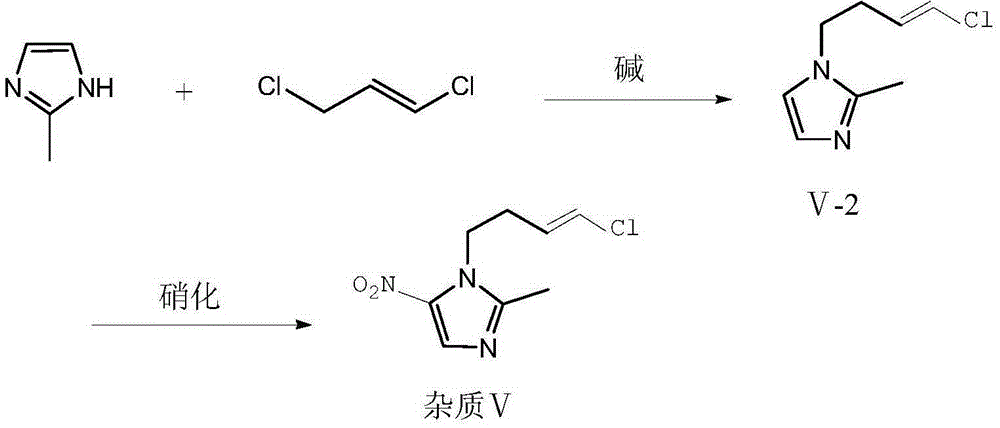
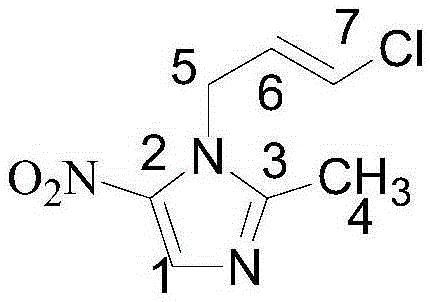
Preparation method of ornidazole injection impurity 1-(3-chloro-propenyl)-2-methyl-5-nitroimidazole
A technology of ornidazole injection and nitroimidazole, which is applied in the field of drug synthesis, can solve problems such as difficulties in the preparation of impurities, and achieve the effects of high yield, good commercial value, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 2-methylimidazole (20.0g, 0.24mol), 1,3-dichloropropene (26.4g, 0.24mol), potassium hydride (12.0g, 0.30mol), and 500ml tetrahydrofuran into a 1L three-necked flask, and heat. Raise the temperature to reflux, keep the temperature and react for 12 hours. After the reaction is completed, filter and spin dry to form an oily substance. Column chromatography (dichloromethane:methanol=50:1, filled with silica gel is 200-300 mesh) yields the intermediate product V-235.5g, Yield: 87.0%.
[0027] Measure 50ml of fuming nitric acid into a 250ml three-necked bottle, cool to 0°C, slowly add the intermediate product (20g, 0.12mol) in the previous step to the system, and control the temperature at 0-5°C. After the addition, 25 g of phosphorus pentoxide was added in batches to the system, and the temperature was controlled at 0-5° C. during the addition. Then keep the reaction at 0-5°C for 4h. After the reaction is complete, pour the reaction solution into purified water cooled ...
Embodiment 2
[0029] Add 2-methylimidazole (20.0g, 0.24mol), 1,3-dichloropropene (33.0g, 0.30mol), potassium carbonate (48.3g, 0.35mol), and 500ml of acetonitrile into a 1L three-necked flask, heat, Raise the temperature to reflux, and keep it warm for 16 hours. After the reaction is completed, filter and spin dry to form an oily substance. Column chromatography (dichloromethane:methanol=50:1, filled with silica gel is 200-300 mesh) yields the intermediate product Ⅴ-233.0g, Yield: 80.8%.
[0030] Measure 50ml of fuming nitric acid into a 250ml three-necked bottle, cool to 0°C, slowly add the intermediate product (20g, 0.12mol) in the previous step to the system, and control the temperature at 0-5°C. After the addition, 30 g of acetic anhydride was slowly added dropwise to the system, and the temperature was controlled at 0-5° C. during the addition. Then the reaction was incubated at 0-5°C for 5h. After the reaction is complete, pour the reaction solution into purified water cooled to 0°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com