Ticagrelor impurity, preparation method and application thereof
A technology of ticagrelor and impurities, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
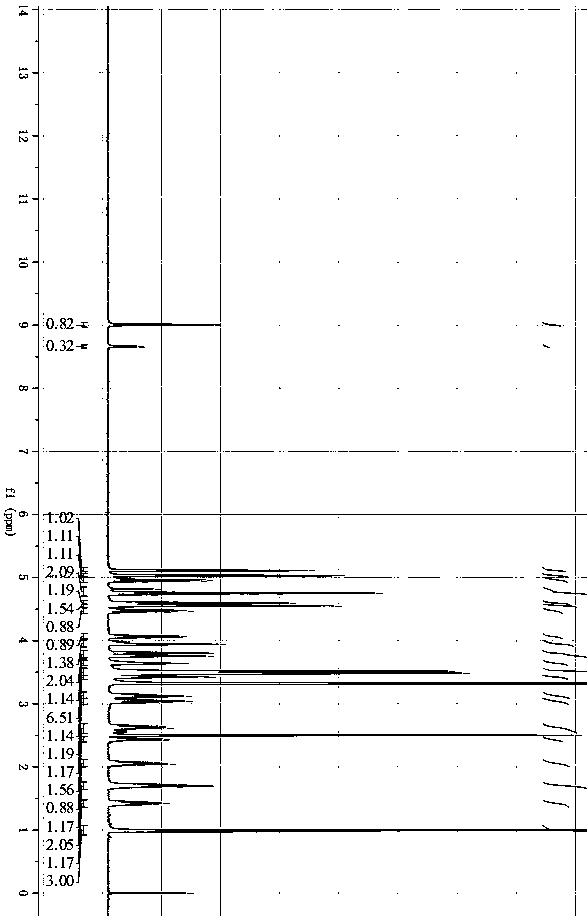
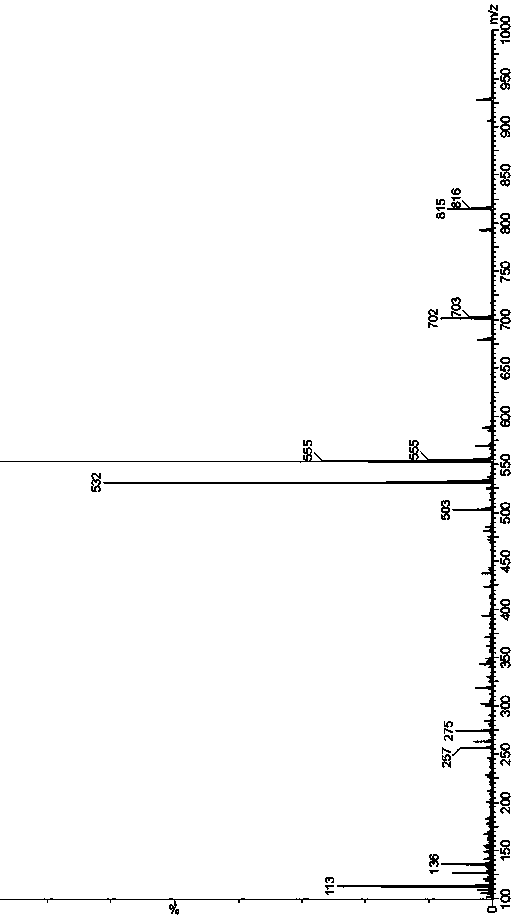
[0060] Example 1 Preparation of ticagrelor impurity 7
[0061]
[0062] 1. Preparation of Impurity 7 Intermediate 1
[0063] 4.70g (12.79mmol) 2-[[(3aR,4S,6R,6aS)-6aminotetrahydro-2,2-dimethyl-4Hcyclopenta1,3-dioxolane- 4-yl]oxy]-ethanol-L-tartrate (SM1), 5.0 g (11.63 mmol) 2-({(3aR,4S,6R,6aS)-6-[7-chloro-5-(propylmercapto )-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyltetrahydro-3aH-cyclopentadieno[d][1 ,3] Dioxolane-4-yl}oxy)ethanol (B) was sequentially added to a 100ml three-necked flask, followed by 43ml of dichloromethane and 7.7ml of N,N-diisopropylethylamine (46.52mmol ), reacted in a 30°C water bath for 24h. After the reaction is over, add 30ml of ice water to the reaction system, separate the liquids, and use the organic phase for later use, extract the water phase twice with 15ml of dichloromethane each time, combine the organic phases; add 30ml of 10% saline solution for washing, and separate the organic phase to 100ml There-necked flask; add 8.0 g of...
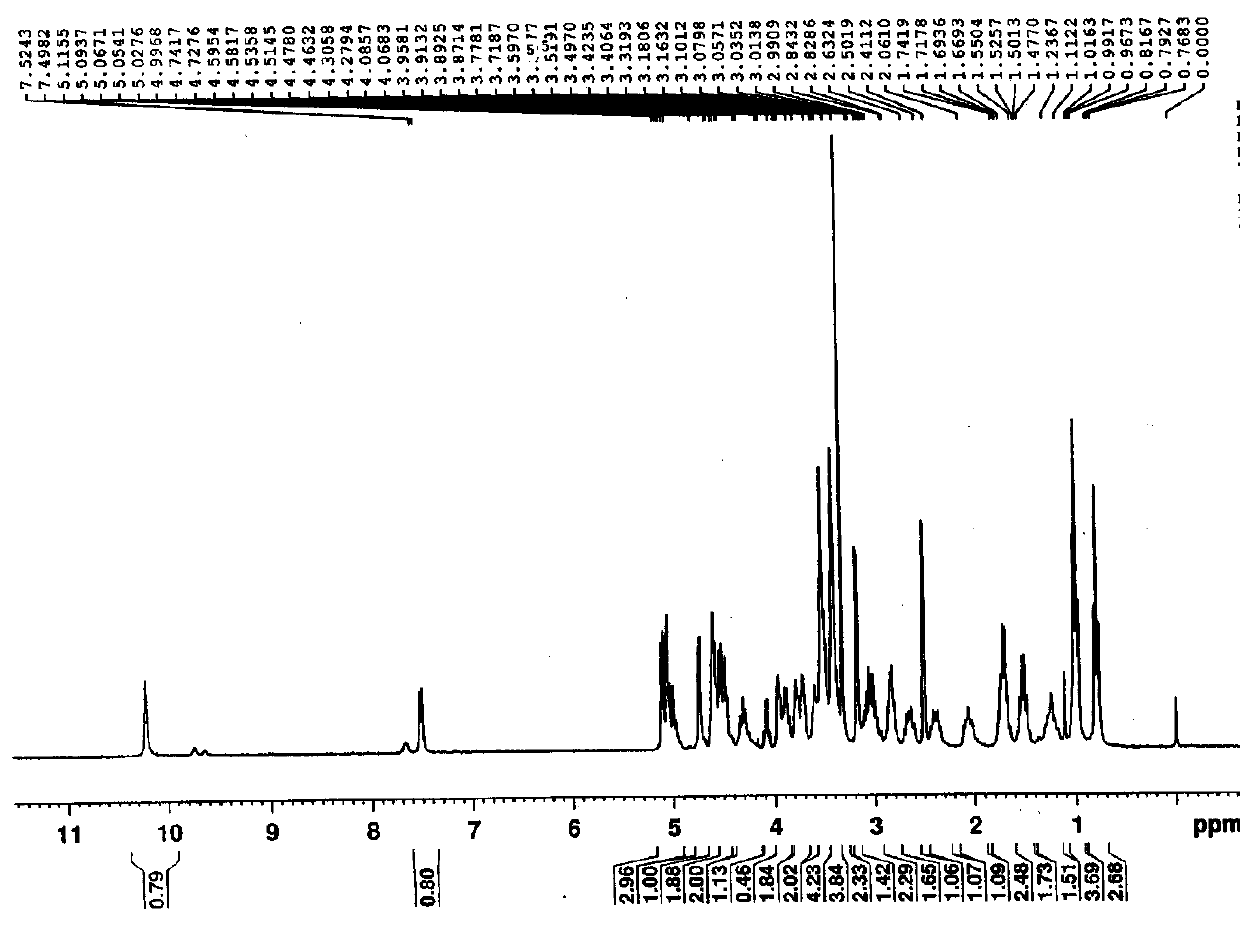
Embodiment 2
[0066] The preparation of embodiment 2 ticagrelor impurity 8
[0067]
[0068] 1. Preparation of Impurity 8 Intermediate 1
[0069] 5.85g (13.96mmol) 2-[((3aR,4S,6R,6aS)-6-{[5-amino-6-chloro-2-(propylthio)pyrimidin-4-yl]amino}-2, 2-Dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]dioxolan-4-yl)oxy]ethanol (A), 5.0 g (11.63 mmol) 2- ({(3aR,4S,6R,6aS)-6-[7-Chloro-5-(propylmercapto)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl ] -2,2-Dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]dioxolan-4-yl}oxy)ethanol (B) was added to a 100ml three-necked flask in sequence 50ml of acetonitrile and 11.37g (34.89mmol) of cesium carbonate were added, and the temperature was raised to 50~60°C for 24h. Cool down to 40~45°C with tap water, add 100ml of purified water, extract 3 times with 70ml of dichloromethane / time, combine the organic phase into a 250ml three-neck flask, add 20g of anhydrous sodium sulfate and stir for 2h, filter, collect the filtrate, and store at 40°C Concentration under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com