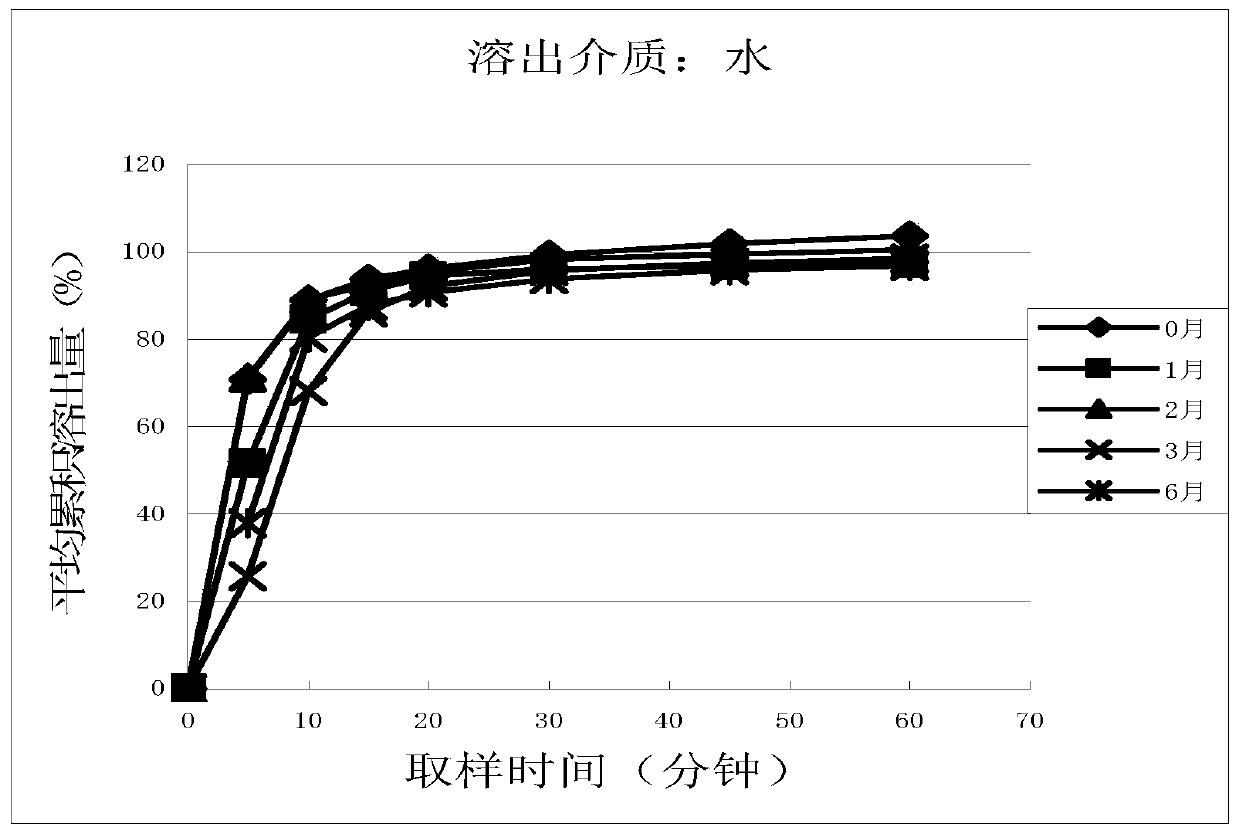
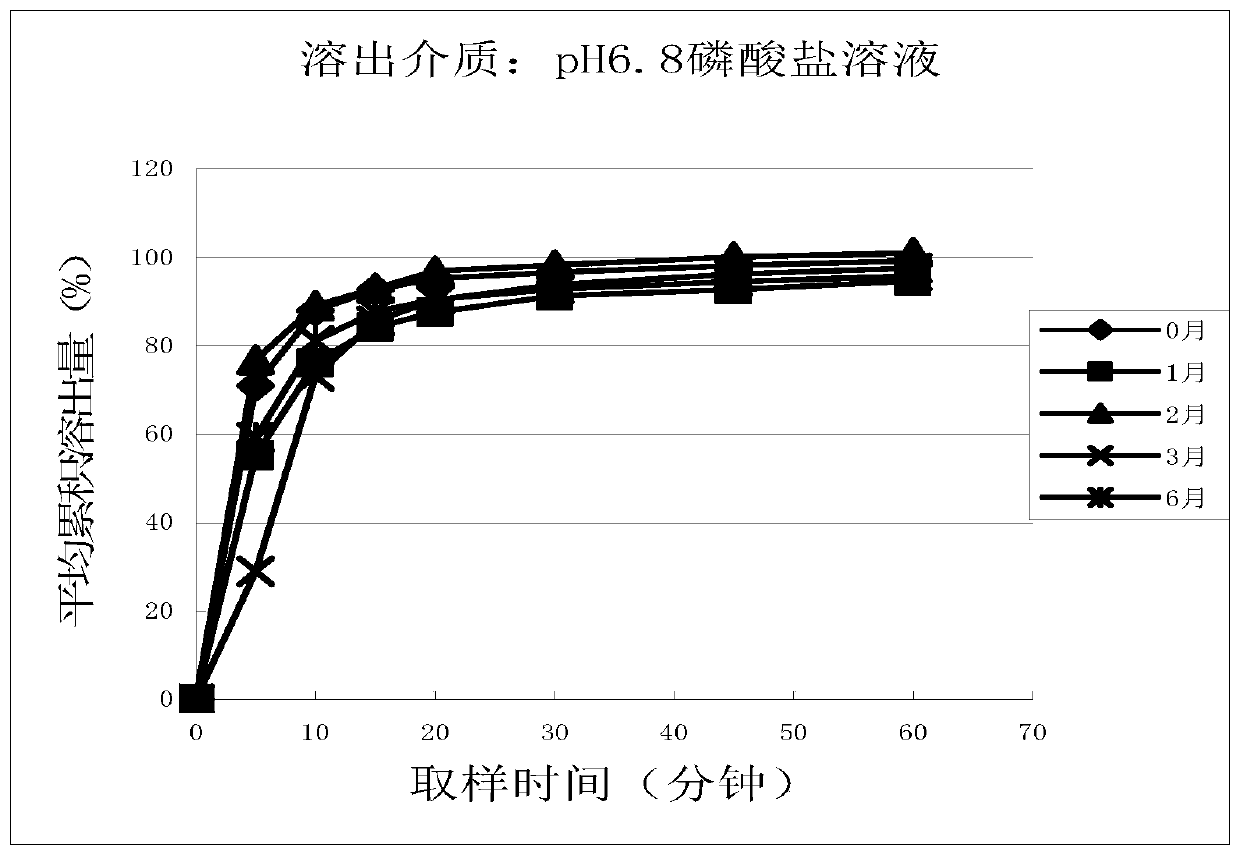
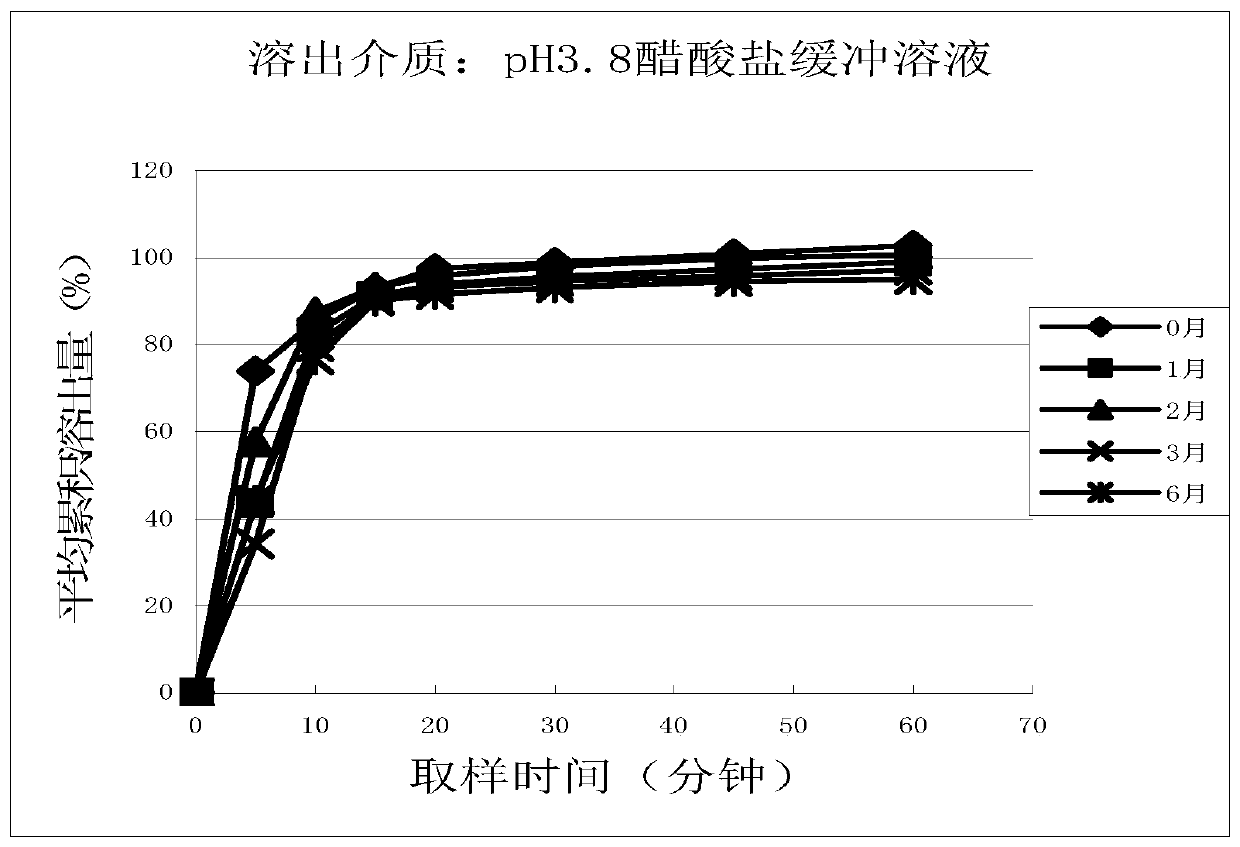
Cetirizine hydrochloride tablet and preparation method thereof
A technology of cetirizine hydrochloride and lizine tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of adhesion, toughness, and isolation effects that do not meet the requirements , unable to meet the quality and storage requirements of cetirizine hydrochloride tablets, and achieve the effects of low water absorption, improved compressibility, and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of cetirizine hydrochloride tablet, in parts by weight, the raw and auxiliary materials are composed as follows:
[0048]
[0049] The preparation method steps are as follows:
[0050] (1) Material preparation process: the raw materials are passed through an 80-mesh sieve, the silicon dioxide is passed through an 80-mesh sieve, and the remaining auxiliary materials are passed through a 60-mesh sieve if there is agglomeration.
[0051] (2) Mixing process: sequentially add microcrystalline cellulose, silicon dioxide Ⅰ, lactose, and cetirizine hydrochloride into the wet granulator, start stirring at 145rpm, cut at 300rpm, mix for 15min, and discharge; Add the powder, silicon dioxide II and magnesium stearate into a three-dimensional mixer, mix for 10 minutes, and discharge.
[0052] (3) Tablet pressing process: According to the content of the main drug in the mixed powder, calculate the theoretical tablet weight, use the GZP-40 tablet press machine, 10.0mm*4.0mm...
Embodiment 2
[0055] A kind of cetirizine hydrochloride tablet, in parts by weight, the raw and auxiliary materials are composed as follows:
[0056]
[0057] The preparation method steps are as follows:
[0058] (1) Material preparation process: the raw materials are passed through an 80-mesh sieve, the silicon dioxide is passed through an 80-mesh sieve, and the remaining auxiliary materials are passed through a 60-mesh sieve if there is agglomeration.
[0059] (2) Mixing process: sequentially add microcrystalline cellulose, silicon dioxide Ⅰ, lactose, and cetirizine hydrochloride into the wet granulator, start stirring at 140rpm, cut at 285rpm and mix for 15min, and discharge; Add silicon dioxide II and magnesium stearate into a three-dimensional mixer, mix for 13 minutes, and discharge.
[0060] (3) Tablet pressing process: According to the content of the main drug in the mixed powder, calculate the theoretical tablet weight, use the GZP-40 tablet press machine, 10.0mm*4.0mm single-s...
Embodiment 3
[0063] A kind of cetirizine hydrochloride tablet, in parts by weight, the raw and auxiliary materials are composed as follows:
[0064]
[0065] The preparation method steps are as follows:
[0066] (1) Material preparation process: the raw materials are passed through an 80-mesh sieve, the silicon dioxide is passed through an 80-mesh sieve, and the remaining auxiliary materials are passed through a 60-mesh sieve if there is agglomeration.
[0067] (2) Mixing process: sequentially add microcrystalline cellulose, silicon dioxide Ⅰ, lactose, and cetirizine hydrochloride into the wet granulator, start stirring at 150rpm, cut at 320rpm and mix for 20min, and discharge; Add silicon dioxide II and magnesium stearate into a three-dimensional mixer, mix for 15 minutes, and discharge.
[0068] (3) Tablet pressing process: According to the content of the main drug in the mixed powder, calculate the theoretical tablet weight, use the GZP-40 tablet press machine, 10.0mm*4.0mm single-s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com