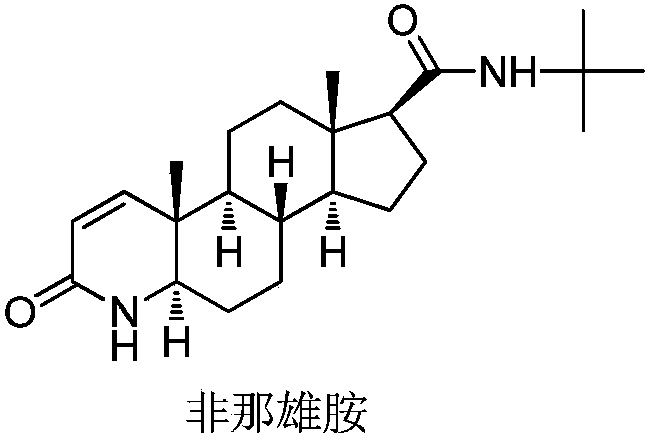
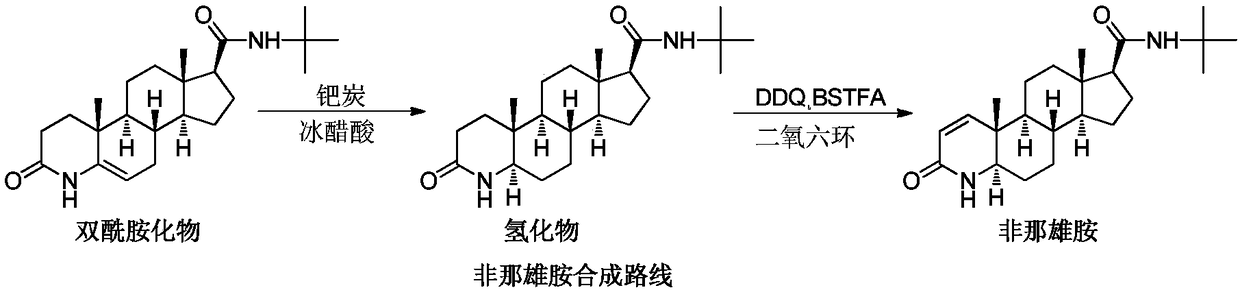
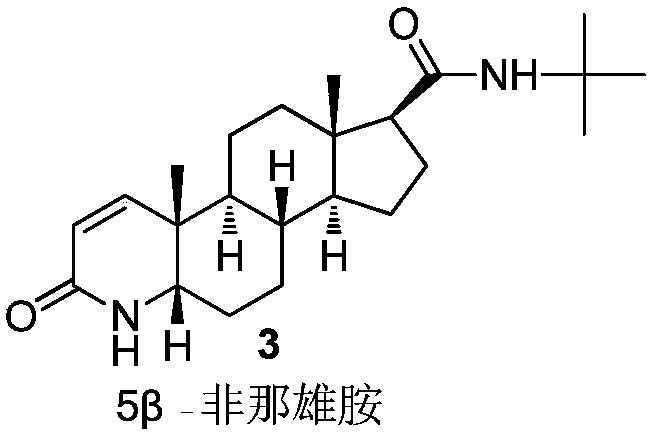
Finasteride chiral impurity (5beta-finasteride) synthesizing and purifying method
A technology of finasteride and chirality, which is applied in the field of chemical synthesis of steroid medicines, and can solve problems such as unreported synthetic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The first step, catalytic hydrogenation: add bisamide (20g, 1W), 5% palladium carbon (10g, 0.5W), p-toluenesulfonic acid (4g, 0.2W) and glacial acetic acid (140g, 7W) in the reaction flask , Nitrogen protection, hydrogen replacement, hydrogen replacement three times, heat preservation at 60 ° C for 8 hours, nitrogen replacement to terminate the hydrogenation reaction. Raise the temperature to 80°C, filter off the palladium carbon while it is hot; concentrate the filtrate to dryness under reduced pressure to obtain a hydride, which is sampled for detection. The main peak content of 5β-chiral hydride in the hydride is 28.8%.
[0044] The second step, refining: add dichloromethane (400ml, 20V) to the hydride, stir to dissolve; sequentially use 10% sodium carbonate solution (180ml, 9V), water (100ml, 5V), water (100ml, 5V) Wash and separate. Collect the dichloromethane layer and concentrate under reduced pressure to a paste, pour into ethyl acetate (200ml, 10V) and concen...
Embodiment 2
[0048] The first step, catalytic hydrogenation: add bisamide (20g, 1W), 5% palladium carbon (10g, 0.5W), p-toluenesulfonic acid (10g, 0.5W) and glacial acetic acid (200g, 10W) in the reaction flask , Nitrogen protection, hydrogen replacement three times through hydrogen insulation 40 ℃ reaction for 12h, nitrogen replacement to terminate the hydrogenation reaction. Raise the temperature to 80°C, filter off the palladium carbon while it is hot; concentrate the filtrate to dryness under reduced pressure to obtain a hydride, which is sampled for detection. The main peak content of 5β-chiral hydride in the hydride is 30.2%.
[0049] The second step, refining: add chloroform (400ml, 20V) to the hydride, stir to dissolve; sequentially use 10% sodium carbonate solution (180ml, 9V), water (100ml, 5V), water (100ml, 5V) Wash and separate. Collect the chloroform layer and concentrate it to a paste under reduced pressure, pour into ethyl formate (200ml, 10V) and concentrate to about (...
Embodiment 3
[0053] The first step, catalytic hydrogenation: add bisamide (20g, 1W), 10% palladium carbon (5g, 0.25W), methanesulfonic acid (1g, 0.05W), glacial acetic acid (100g, 5W) in the reaction flask, Nitrogen protection, hydrogen replacement, hydrogen replacement three times, heat preservation at 80°C for 6 hours, nitrogen replacement to terminate the hydrogenation reaction. The palladium carbon was filtered off while it was hot; the filtrate was concentrated to dryness under reduced pressure to obtain a hydride, which was sampled for detection. The main peak content of 5β-chiral hydride in the hydride is 26.1%.
[0054] The second step, refining: add dichloromethane (400ml, 20V) to the hydride, stir to dissolve; sequentially use 10% sodium carbonate solution (180ml, 9V), water (100ml, 5V), water (100ml, 5V) Wash and separate. Collect the dichloromethane layer and concentrate it to a paste under reduced pressure, pour in isopropyl acetate (200ml, 10V) and concentrate to about (120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com