Lisinopril impurity and preparation method thereof
A technology of lisinopril dihydrate and compound, which is applied in the field of medicinal chemistry, can solve problems such as difficult removal of impurities and difficulty in obtaining finished products of lisinopril, and achieve a strong practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
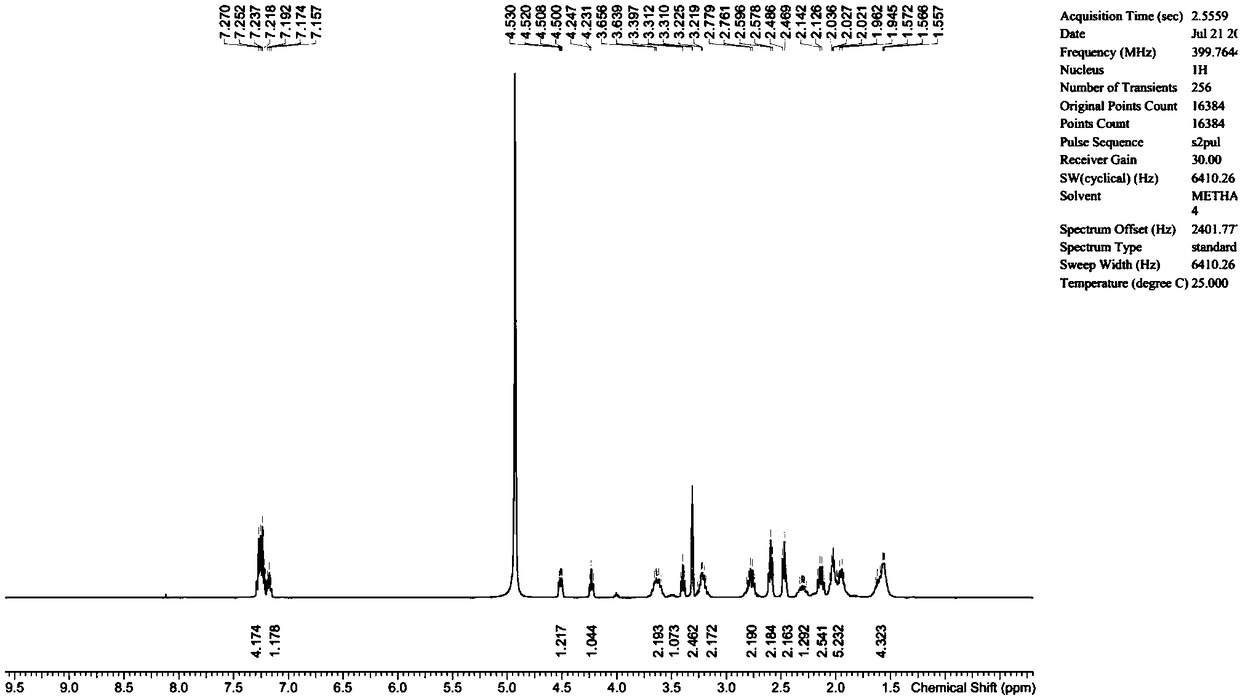
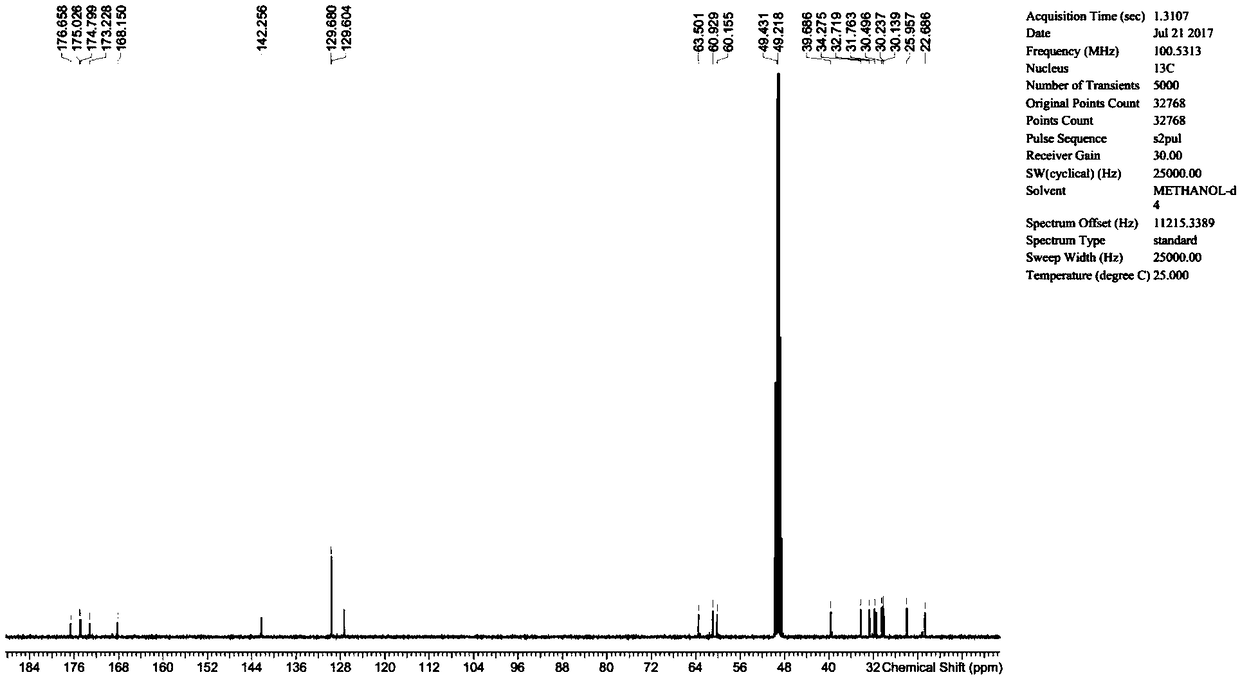
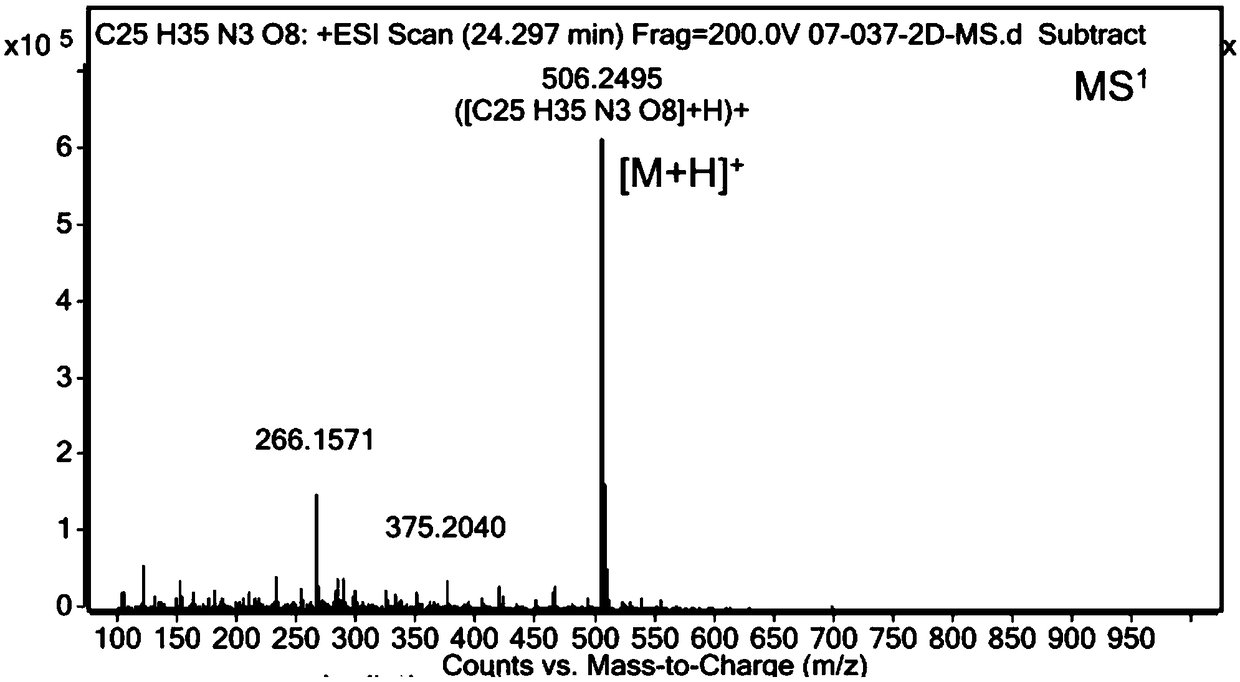
[0046] Add 1g of lisinopril anhydrous to 20mL of methanol, add 0.30g of sodium hydroxide and 0.30g of succinic anhydride, then add 20mL of water, heat up to 45°C and react for 18 hours, then adjust the reaction with 1N dilute hydrochloric acid solution Liquid pH is between 2.5-3.5, and rotary evaporator removes moisture and organic solvent, and the obtained solid is detected by HPLC, and the purity is 65.6%, and then column chromatography method [HP-Silica normal phase silica gel, eluent is Dichloromethane:methanol=(10:1, V / V)] the target product is separated to obtain N 6 -(3-carboxypropionyl)lisinopril 0.64g, HPLC purity 99.0%, yield 51%.
Embodiment 2
[0048] Add 1 g of lisinopril dihydrate into 30 mL of water, then add 0.42 g of potassium hydroxide and 0.32 g of succinic anhydride, heat up to 55°C and react for 12 hours, then adjust the pH of the reaction solution to 3-4 with 1N dilute hydrochloric acid solution In between, the obtained reaction solution was detected by HPLC, and the purity was 72.1%, and then freeze-dried to remove the moisture in the reaction system, and the obtained solid was subjected to column chromatography [HP-Silica normal phase silica gel, and the eluent was dichloro Methane:methanol=(12:1, V / V)] the target product is separated to obtain N 6 -(3-carboxypropionyl)lisinopril 0.70 g, HPLC purity 99.6%, yield 61%.
Embodiment 3
[0050] Add 1 g of lisinopril dihydrate into 30 mL of water, then add 0.86 g of potassium hydroxide and 0.35 g of succinimide, heat up to 55°C for 12 hours, and then adjust the pH of the reaction solution to 3 with 1N dilute hydrochloric acid solution Between -4, the obtained reaction solution is detected by HPLC, and the purity is 69.0%, and then freeze-dried to remove the moisture in the reaction system, and the obtained solid is used for column chromatography [HP-Silica normal phase silica gel, and the eluent is Dichloromethane:methanol=(12:1, V / V)] the target product is separated to obtain N 6 -(3-carboxypropionyl)lisinopril 0.66g, HPLC purity 99.4%, yield 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com