Parecoxib sodium degradation impurity and preparation, detection method and application thereof
A parecoxib sodium and impurity technology, which is applied in the field of drug synthesis, can solve the problem of parecoxib sodium degrading less impurities, and achieve the effect of accurate results and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment provides the preparation of the compound of formula I, and its reaction formula is as follows:
[0039]
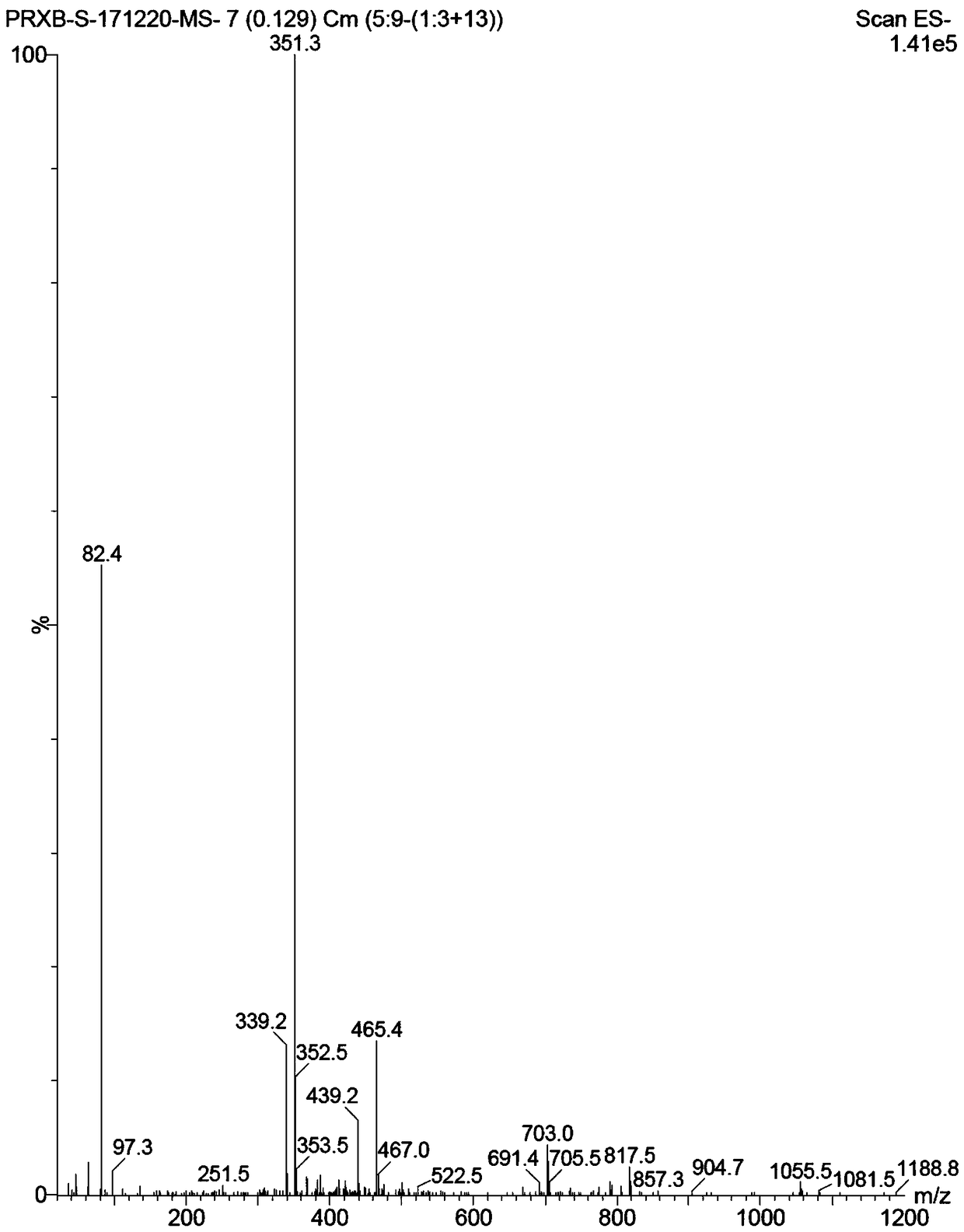
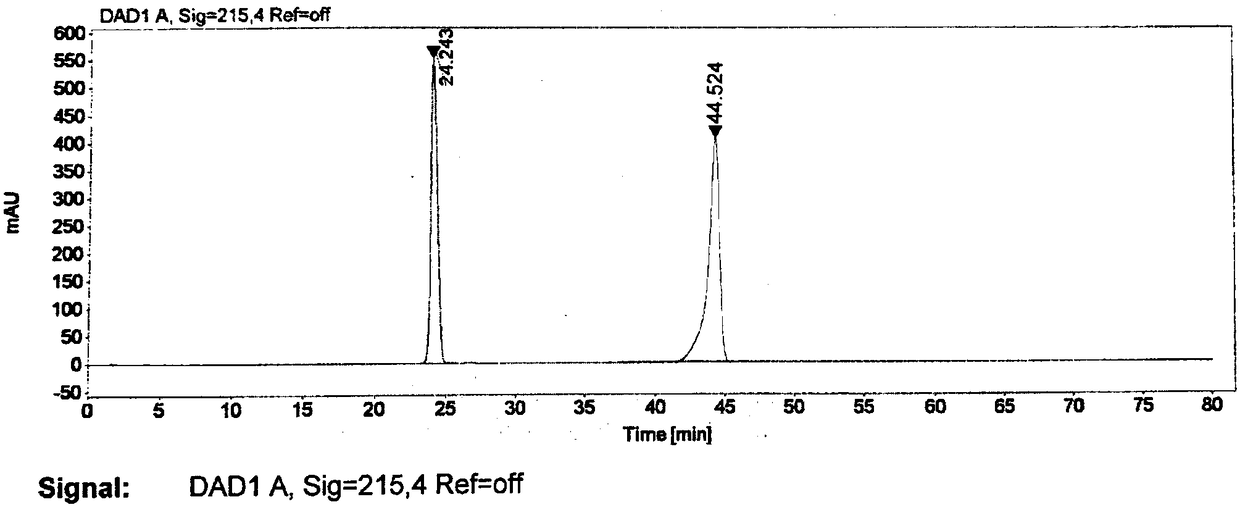
[0040] The specific operation is: sequentially add 2.5g of the compound of formula II and 30mL of dry tetrahydrofuran into the round bottom flask, slowly add 11mL of n-butyllithium (1.6M) into the reaction solution under the protection of nitrogen and at a low temperature of -78°C, after the addition is complete, , continue to stir the reaction for 2h; the reaction solution was slowly warmed to room temperature and reacted overnight. Slowly add saturated ammonium chloride solution to the reaction solution to quench the reaction, extract with ethyl acetate, dry and concentrate the organic layer, and obtain the compound of formula I (R=H) by column chromatography as a white solid, about 0.56 g, and obtain Yield: 24%; HPLC purity: 96.3%.
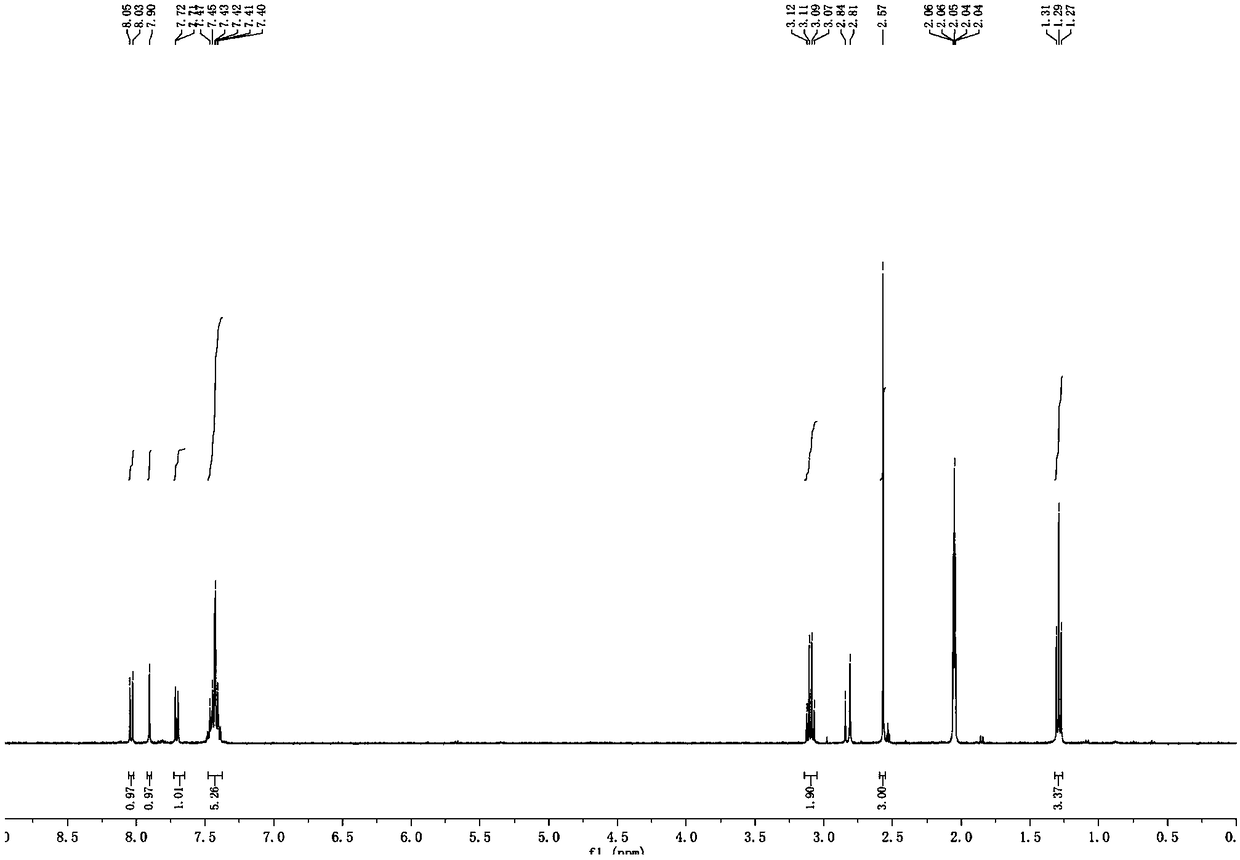
[0041] Formula I compound: 1 H-NMR (CD 3 COCD 3 ,400MHz): δ=8.04(d, J=8.0Hz, 1H), 7.90(s, 1H), 7.72(d, J=4.0...
Embodiment 2
[0044] The present embodiment provides the preparation of the compound of formula III, and its reaction formula is as follows:
[0045]
[0046] The specific operation is: sequentially add 4.5g of 4-bromo-2-methoxycarbonylbenzenesulfonamide (preparation method reference Bioorganic & Medicinal Chemistry Letters 19 (2009) 6855-6861), 40mL of dry tetrahydrofuran into the round bottom flask, nitrogen protection conditions Lower the temperature to 0°C, slowly drop 45mL of ethylmagnesium bromide solution (1.0M, tetrahydrofuran solution) into the reaction solution, after the drop is completed, continue stirring for 30 minutes, then slowly warm the reaction solution to room temperature, and stir the reaction overnight . 20 mL of water was added to the reaction solution to quench the reaction, extracted with ethyl acetate, the organic layer was dried and concentrated, and the residue was purified by column chromatography to obtain 1.5 g of the compound of formula III.
Embodiment 3
[0048] This embodiment provides the preparation of the compound of formula I, and its reaction formula is as follows:
[0049]
[0050] In Example 2, 1.5g of the compound of formula III, 1.7g of pinacol diborate, 0.3g of [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, 1.2g of potassium acetate, 20ml Add 1,4-dioxane into a round bottom flask, under nitrogen protection, slowly raise the temperature of the reaction solution to 100°C for 5 hours, cool the reaction solution to room temperature, filter, concentrate the filtrate, and purify the residue by column chromatography followed by subsequent reactions.
[0051] The above product, 1.2g of compound of formula IV, 0.2g of tetrakis(triphenylphosphine)palladium, 10mL of ethanol, and 5mL of 2M sodium carbonate solution were successively added into a round bottom flask, and the temperature was slowly raised to reflux under stirring. The progress of the reaction was monitored by TLC. The reaction solution was cooled to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com