Preparation method of tofacitinib impurity
A tofacitinib, preparation process technology, applied in the direction of organic chemistry, etc., can solve the problems of difficult to effectively obtain compound I and compound, easy to produce other impurities, etc., and achieve the effect of easy separation and purification, high product purity, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
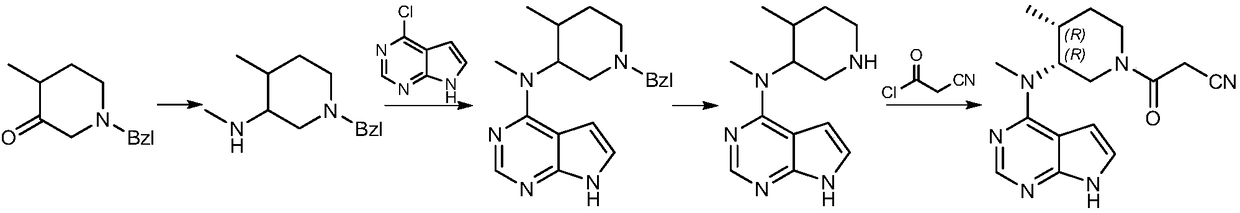
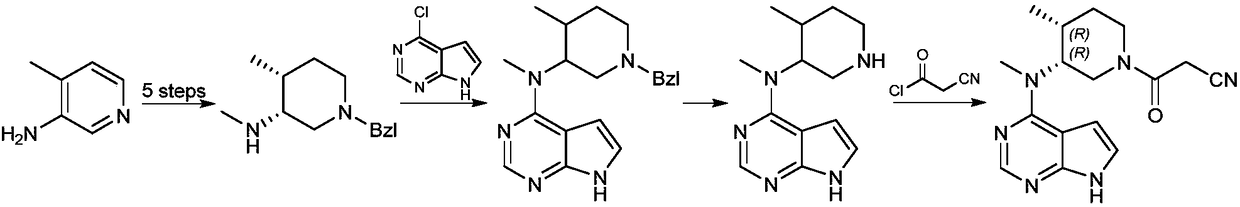
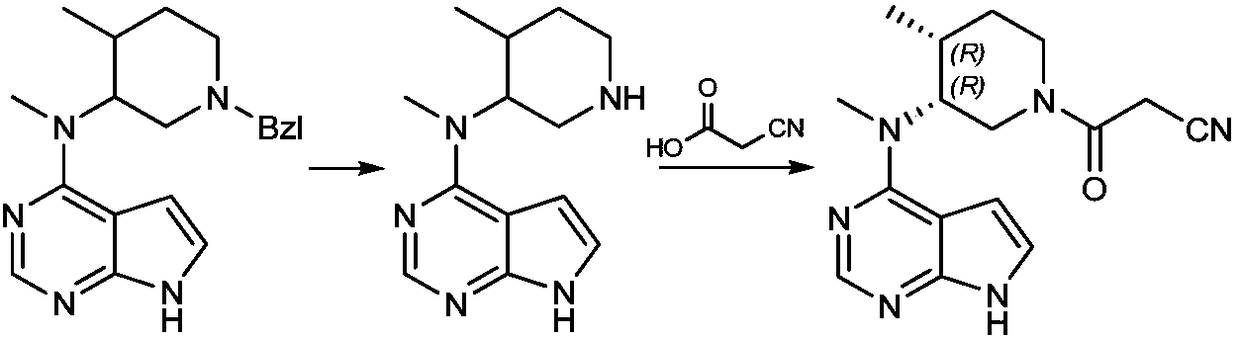
[0032] A preparation method of tofacitinib related substances, the structural formula of tofacitib related substances is shown in formula I, The synthetic route is as follows:
[0033]
[0034] Including the following steps:
[0035] 1) Dissolve tofacitib in an acid solution with a mass concentration of 50%-60%, the acid being one of sulfuric acid and methanesulfonic acid;
[0036] 2) Raise the temperature to 40~50℃ to react;
[0037] 3) End the reaction, neutralize, extract, and crystallize to obtain the tofacitib related substances represented by formula I.
[0038] As a further improvement of the above preparation method, the mass concentration of the acid used in the preparation process of the compound of formula I is 50%-60%.
[0039] As a further improvement of the above preparation method, the mass concentration of the acid used in the preparation process of the compound of formula I is 55%.
[0040] As a further improvement of the above preparation method, the reaction temperatur...
Embodiment 1
[0062] 1) Add 3.12g (10mmol) of tofacitib and sulfuric acid (55%, 75ml) into the reaction flask, heat to 50°C and stir for 7-8 hours;
[0063] 2) HPLC confirms that the reaction is complete, cool to 5-10°C, add sodium hydroxide solution (10%) to neutralize to pH 8-9;
[0064] 3) Extract three times with dichloromethane (50ml×3);
[0065] 4) After drying, filtering, and concentrating the extract, methyl tert-butyl ether (25 ml) was added, and an off-white solid (compound I, 1.34 g) was precipitated out with slow stirring. The yield was 40.5%.
[0066] After testing, the purity of the off-white solid is 99.0%; the mass spectrum and nuclear magnetic data are as follows:
[0067] ESI-MS: m / z(M+H + )=331.2;
[0068] 1 HNMR(500MHz, DMSO-d6), δ(ppm): 1.00~1.02(d,5H), 1.53~1.84(m,2H), 2.34~2.43(m,1H), 3.21~3.32(m,5H), 3.50~3.52(t,1H), 3.62~3.71(m,1H), 3.77~3.92(d,1H), 6.55(s,1H), 6.95~7.02(d,1H), 7.13~7.14(d,1H) ), 7.42~7.49(d,1H), 8.10~8.12(d,1H), 11.64~11.70(d,1H);
[0069] 13 CNMR (125MHz, D...
Embodiment 2
[0071] Add 3.12g (10mmol) of tofacitib and methanesulfonic acid (50%, 80ml) into the reaction flask, heat to 50°C and stir to react for 12 hours. After HPLC confirms that the reaction is complete, the temperature is reduced to 5-10°C, and sodium hydroxide is added The solution (10%) was neutralized to pH 8-9, and extracted three times with dichloromethane (50ml×3); the extract was dried, filtered and concentrated, then methyl tert-butyl ether (25ml) was added, and an off-white solid was precipitated out under slow stirring. (Compound I, 1.00 g, purity 98.2%), the yield is 30.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com