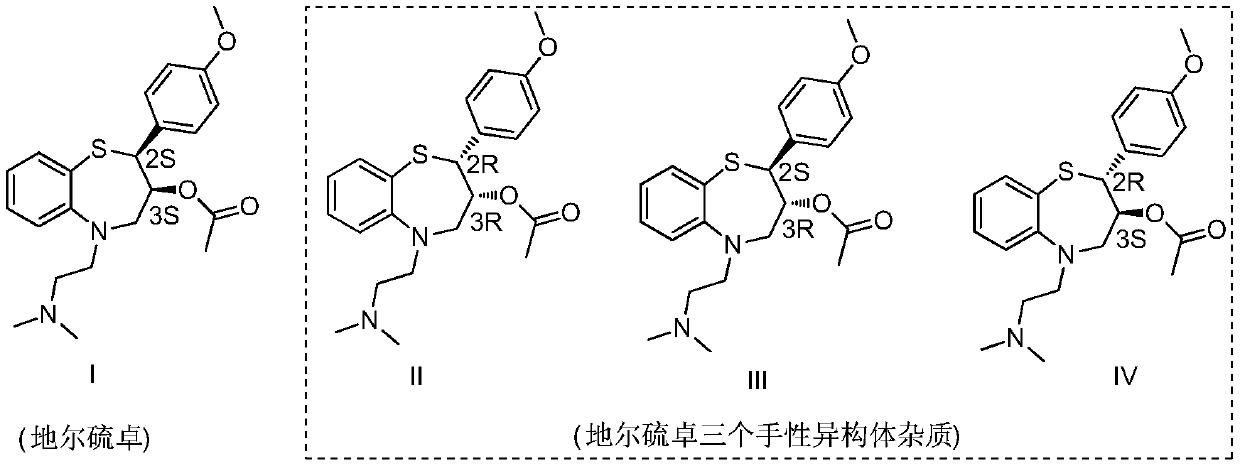
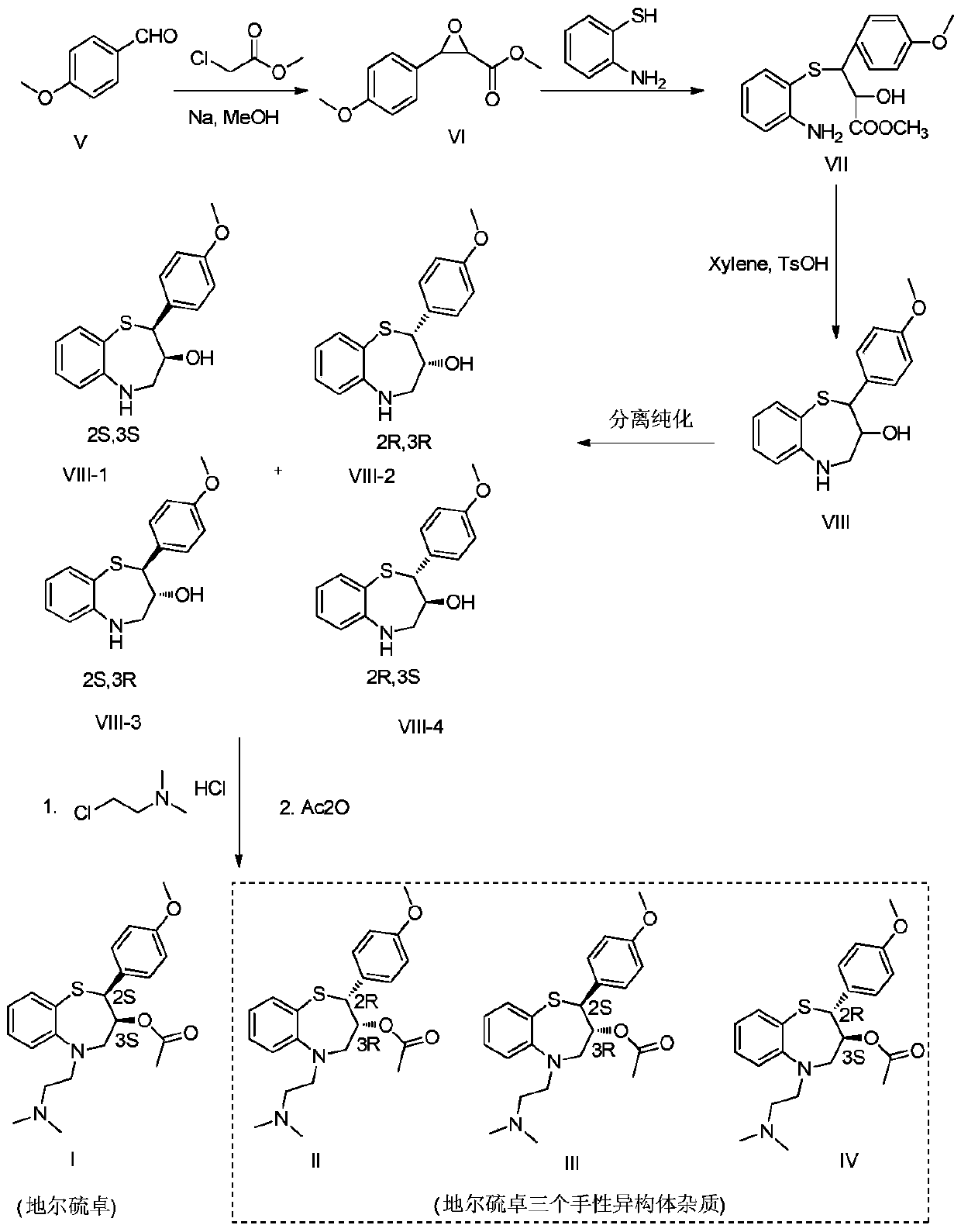
Preparation and purification method of diltiazem chiral isomer impurity
A technology of chiral isomer and diltiazem, applied in the field of medicine, can solve the problems of long time consumption, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 500mL of methanol into a 1L four-neck flask, under nitrogen protection, add 6g of sodium in batches under stirring at 0°C, then slowly add 27.2g of p-methoxybenzaldehyde and 28.5g of methyl chloroacetate dropwise at a controlled temperature of 0-10°C, After the dropwise addition, it was raised to 25°C for reaction. After the reaction was completed, it was poured into 1L of ice water, and a large amount of white solid was precipitated, which was filtered, washed, and dried to obtain 28 g of white solid VI.
[0019] Add 20 g of the obtained white solid VI into a 1L reaction flask, add 500 mL of toluene, add 14.4 g of o-aminothiophenol and 1.5 g of calcium chloride, heat up to 50 ° C for 24 hours, cool down to room temperature after the reaction, and filter to remove the solid For the insoluble matter, the toluene layer was extracted twice with 100 mL of concentrated hydrochloric acid, and the concentrated hydrochloric acid layer was added to 1 L of water under ice coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com