Impurities of R-lipoic acid or tromethamine salts thereof, preparation method for impurities and detection method for impurities
A technology of d-lipoic acid and impurity reference substances, applied in the field of medicinal chemistry and analysis, to achieve the effect of accurate and applicable analysis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
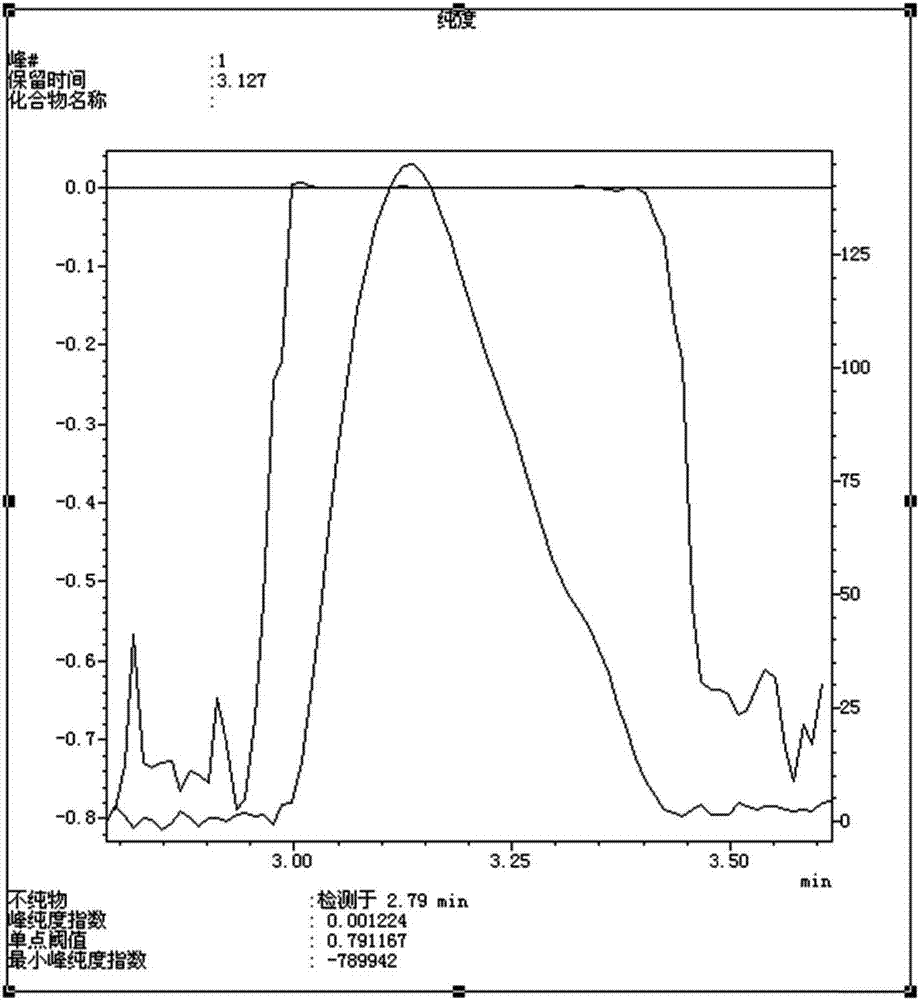
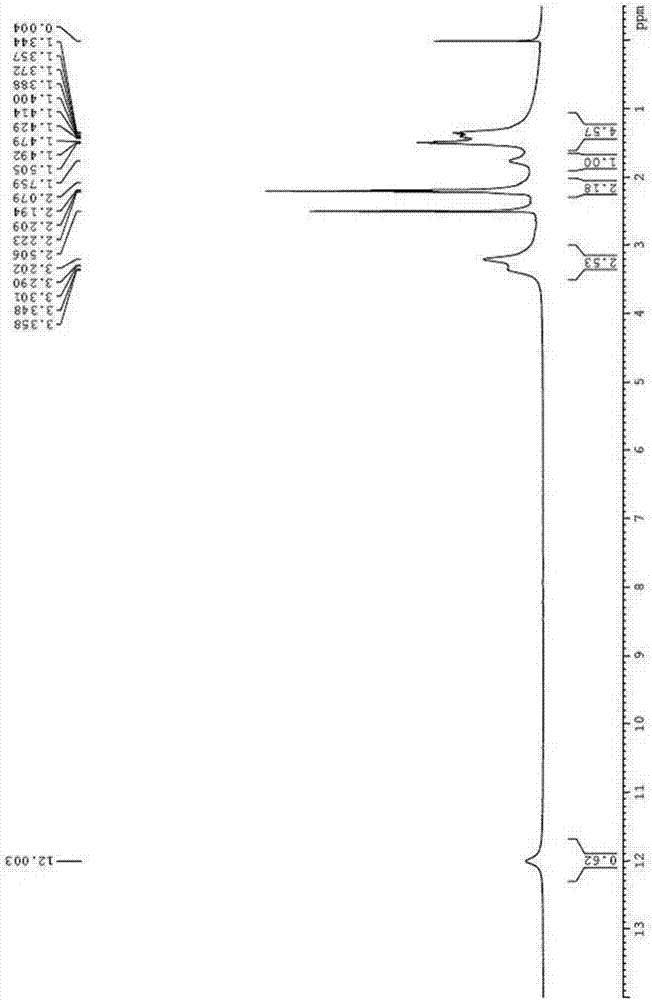
[0054] Example 1: Preparation of (R)-5-(1,2-dithiopentan-3-yl)pentanoic acid-2,2-dioxide (compound of formula I)
[0055] Dissolve 60g of D-lipoic acid tromethamine in 1000ml of purified water to prepare a 0.06g / ml solution, and irradiate the aqueous solution with 4500lx±500lx strong light for 15 days to obtain a primary preparation sample solution.
[0056] Chromatographic conditions for HPLC primary preparation:
[0057] Instrument: Innovative Tongheng preparative liquid phase LC6000, preparative column DAC100, diameter 10cm
[0058] Stationary phase: Octadecylsilane bonded silica gel, 1.5kg charge, 10μm
[0059] Mobile phase: A-0.15% phosphoric acid aqueous solution, B-acetonitrile
[0060] Detection wavelength: 215nm
[0061] Flow rate: 200ml / min
[0062] Column temperature: 25°C
[0063] Injection volume: 200ml
[0064] Elution method: Gradient elution
[0065]
[0066]Collect the substance corresponding to the target peak at a retention time of about 50-60min. ...
Embodiment 2
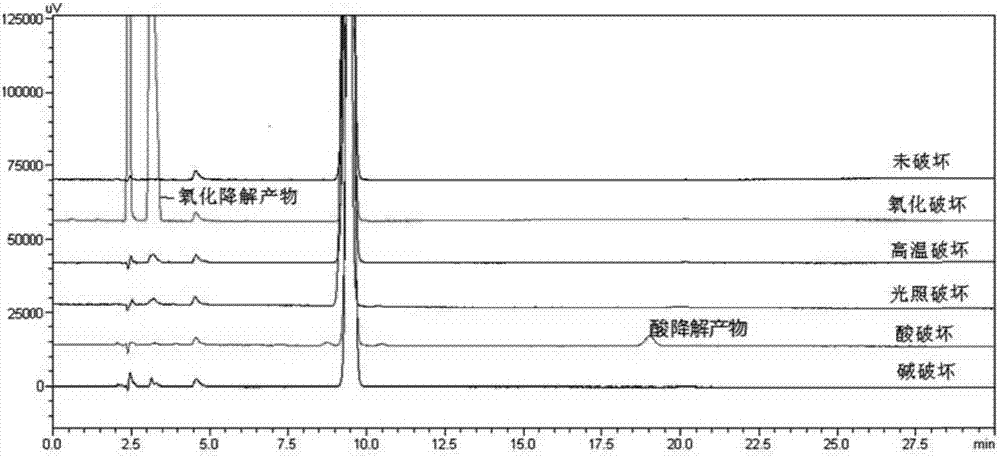
[0089] Example 2: Separation and detection of related substances of D-lipoic acid tromethamine according to the method included in USP
[0090] This embodiment investigates the separation effect of the main component and related substances in the D-lipoic acid trometamol sample by the method included in USP.
[0091] Preparation of each sample of D-lipoic acid tromethamine:
[0092] Sample 1 (undestroyed): Take about 15 mg of D-lipoic acid tromethamine, weigh it accurately, put it in a 10 ml measuring bottle, dissolve it with mobile phase and dilute to the mark, and shake well.
[0093] Sample 2 (oxidative damage): Take about 15 mg of D-lipoic acid tromethamine, accurately weigh it, put it in a 10 ml measuring bottle, add 1 ml of 3% hydrogen peroxide solution to dissolve, immediately dissolve and dilute to the mark with mobile phase, shake uniform.
[0094] Sample 3 (high temperature destruction): Take about 15mg of D-lipoic acid tromethamine, weigh it accurately, put it in ...
Embodiment 3
[0109] Embodiment 3: Separation and detection of D-lipoic acid tromethamine related substances according to the method of the present invention
[0110] This embodiment investigates the separation effect of the method of the present invention on the main component and related substances in the D-lipoic acid trometamol sample.
[0111] Preparation of each sample of D-lipoic acid tromethamine:
[0112] Sample 1 (undamaged): Take about 15 mg of D-lipoic acid tromethamine, accurately weigh it, put it in a 10ml measuring bottle, dissolve it with a diluent [0.05% phosphoric acid solution-acetonitrile (85:15)] and dilute to the mark , shake well.
[0113] Sample 2 (oxidative damage): Take about 15 mg of D-lipoic acid tromethamine, accurately weigh it, put it in a 10 ml measuring bottle, add 1 ml of 3% hydrogen peroxide solution to dissolve, and immediately add diluent [0.05% phosphoric acid solution-acetonitrile (85:15)] diluted to the mark and shaken well.
[0114] Sample 3A (hig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com