Reference substance of micurium chloride and preparation method thereof
A technology of Micuronium chloride and reference substance, applied to the reference substance of Micuronium chloride and the field of preparation thereof, can solve problems such as being unfavorable to be used as a reference substance, lack of chemical stability, difficulty in quality research and the like, and achieve non-degradable, Low cost and reasonable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
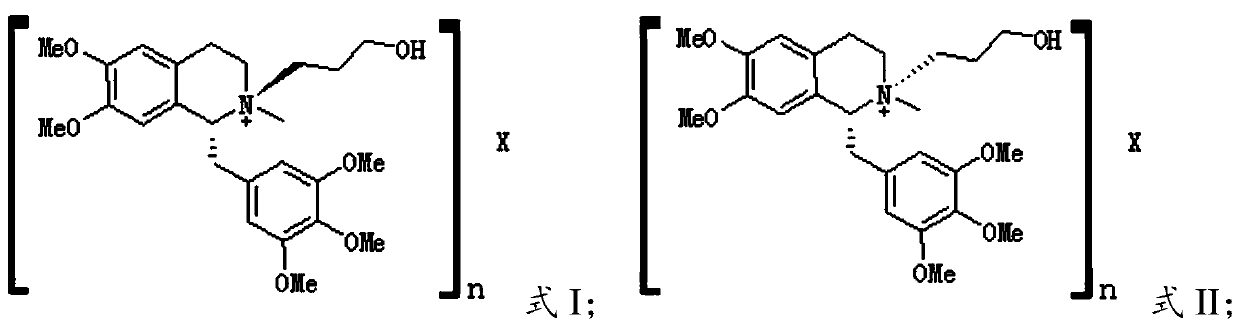
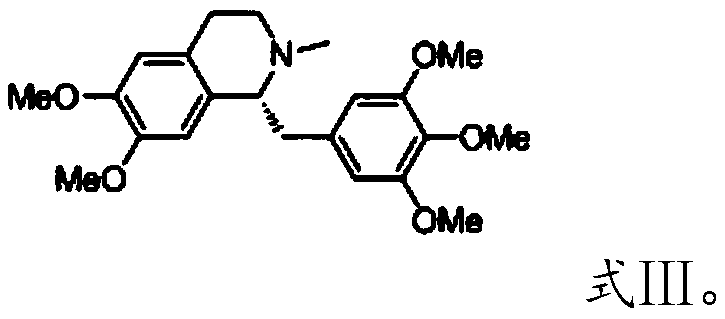
[0039] The present invention provides the preparation method of the reference substance of mivacurium chloride described in the above technical scheme, and when X does not exist, the preparation method comprises the following steps:
[0040] (1) carrying out the first quaternization reaction with the compound having the structure shown in formula III and 3-chloropropanol under the catalysis of sodium carbonate, to obtain the first quaternization product;
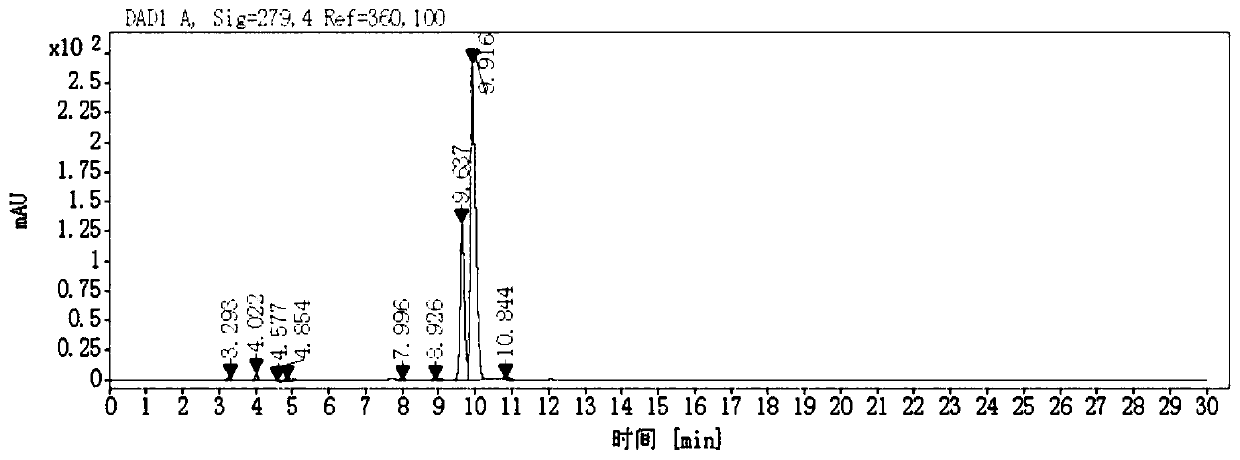
[0041] (2) The first quaternized product was separated by preparative liquid chromatography to obtain the reference substance represented by formula I and the reference substance represented by formula II respectively.
[0042]
[0043] When X does not exist, the present invention performs the first quaternization reaction on the compound having the structure shown in formula III and 3-chloropropanol under the catalysis of sodium carbonate to obtain the first quaternization product. In the present invention, the first qua...
Embodiment 1
[0080] Preparation of quaternized product (X is absent):
[0081]
[0082] Dissolve 0.3 mol of compound III in 500 mL of 2-butanone, add 0.6 mol of 3-chloropropanol, stir to dissolve, and finally add 0.12 mol of sodium carbonate. Turn on the heating, and keep at reflux for 18 hours at 80°C. After the reaction, cool to an internal temperature of 50-60°C and heat filter to obtain a light red filtrate. Concentrate to dryness under reduced pressure to obtain a viscous substance. After adding purified water and stirring to dissolve, extract with dichloromethane, and take the aqueous phase. After sodium carbonate was added to the aqueous phase, it was extracted twice with dichloromethane, and the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain yellow solid IV (X was absent). ESI(-): m / z 446.2.
Embodiment 2
[0084] Quaternized product (X is I - ) preparation:
[0085]
[0086] Dissolve 0.3 mol of compound III in 800 mL of 2-butanone, add 0.9 mol of 3-chloropropanol and 0.9 mol of sodium iodide, stir to dissolve, and finally add 0.18 mol of sodium carbonate. Turn on the heating, and keep at reflux for 18 hours at 76°C. After the reaction is over, cool to an internal temperature of 50-60°C and heat filter to obtain an orange-red filtrate. Concentrate to dryness under reduced pressure to obtain a viscous substance. After adding purified water and stirring to dissolve, extract with dichloromethane, and take the aqueous phase. Add sodium iodide, stir and dissolve, extract twice with dichloromethane, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate to dryness under reduced pressure to obtain a yellow solid V (X is I - ). ESI(-): m / z 446.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com