Vortioxetine hydrobromide crystalline form C and preparation method thereof
A technology of vortioxetine hydrobromide and crystal form, applied in the field of medicinal chemistry, can solve the problems of crystal form stability, unsatisfactory preparation method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Put 5.0 g of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine and 50 ml of DMF in a reaction flask, heat and stir to dissolve the solid completely, remove the heating bath, cool to room temperature and wait use;
[0058] Dissolve 625g of ammonium bromide in 1500ml of deionized water with stirring at room temperature, filter out mechanical impurities, add the filtrate to a 3L three-necked flask, cool it down to an internal temperature of 20-25°C while stirring, and add the above solution dropwise (the dropwise addition is completed in 5 minutes) , stirred at 20-25°C for 1.5 hours, filtered with suction, stirred and washed the solid with 150ml of 0°C water, filtered with suction, and washed with 20ml of 0°C water for 4 times to obtain a white solid. Drying under pressure for 5 hours gave 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine hydrobromide hemihydrate crystal form C.
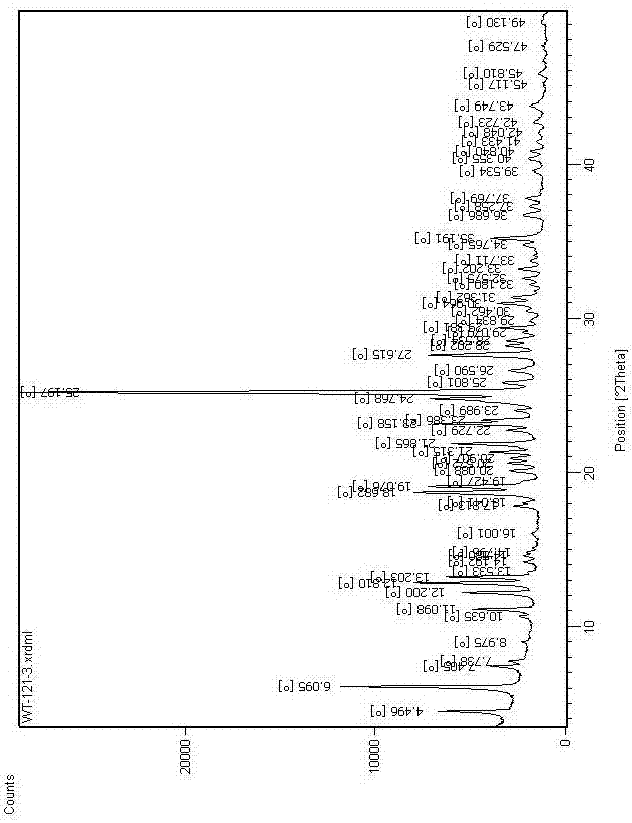
[0059] The prepared crystal form C was subjected to powder diffraction analysis, different...
Embodiment 2
[0063] Put 5.0 g of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine and 50 ml of DMF in a reaction flask, heat and stir to dissolve the solid completely, remove the heating bath, cool to room temperature and wait use;
[0064] Dissolve 625g of ammonium bromide in 1500ml of deionized water with stirring at room temperature, filter out mechanical impurities, add the filtrate into a 3L three-necked flask, cool it to the inner temperature of 0-5°C under stirring, and add the above solution dropwise (the dropwise addition is completed in 5 minutes) , stirred at 0-5°C for 15 hours, filtered with suction, stirred and washed the solid with 150ml of water at 0°C, filtered with suction, and washed with 20ml of water at 0°C for 4 times to obtain a white solid, which was air-dried at room temperature for 20 hours, then reduced to 40°C. Dry under pressure for 5 hours 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine hydrobromide hemihydrate crystal form C.
[0065] After testing and v...
Embodiment 3
[0067] Dissolve 625g of ammonium bromide in 1500ml of deionized water with stirring at room temperature, filter out mechanical impurities, add the filtrate to a 3L three-necked flask, cool it to an internal temperature of 5-10°C while stirring, and add solid powder 1-[2-(2, 5.0g of 4-dimethylphenylsulfanyl)phenyl]piperazine, stirred at 5-10°C for 48 hours, filtered with suction, stirred and washed the solid with 150ml of water at 0°C, filtered with suction, and then washed with 20ml of water at 0°C×4 After washing twice, a white solid was obtained, which was air-dried at room temperature for 20 hours, and then dried under reduced pressure at 40°C for 5 hours. 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine hydrobromide Compound Form C.
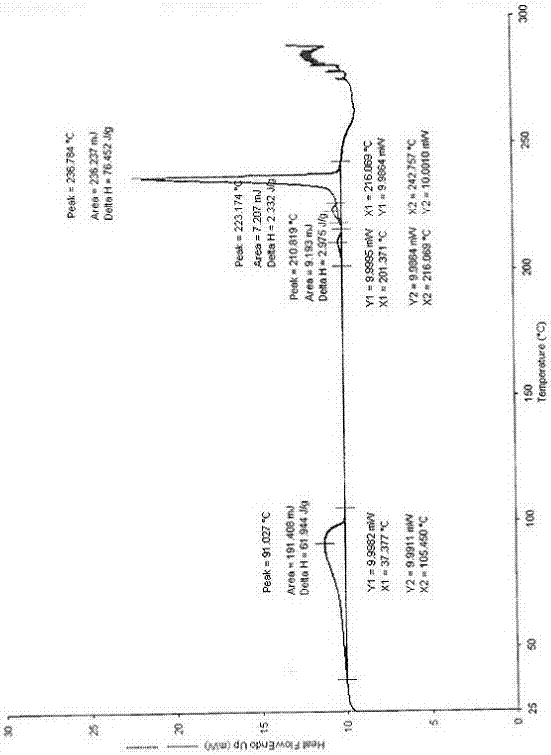
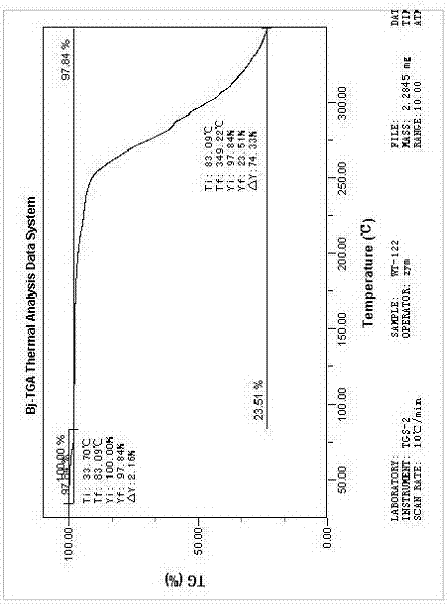
[0068] After testing, its X-ray powder diffraction pattern is as follows: figure 1 The position fluctuations of all characteristic peaks are within the error range, and its DSC spectrum and TGA spectrum are also consistent with figure 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com