Vortioxetine hydrobromide tablets and preparation method thereof
A technology of vortioxetine hydrobromide and hydrobromic acid, which is applied in the field of medicine, can solve the problems of not being able to most effectively improve the efficiency of large-scale industrial production, the time-consuming granulation process, and the influence of drug stability, so as to save production costs , Solve light instability, good stability effect
Active Publication Date: 2015-12-30
KAMP PHARMA
View PDF4 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0003] Chinese Patent Publication No. CN104644594A discloses a vortioxetine hydrobromide stomach-dissolving tablet and a preparation method thereof, the raw materials of which include vortioxetine hydrobromide, a lubricant, a filler, a binder and a disintegrant; its preparation The method includes steps such as granulation, and the granulation process takes a long time. In this process, the drug is exposed to light, which easily degrades the raw materials, which has a certain impact on the stability of the drug, and the production cycle is long, so it cannot be most effectively improved. Efficiency of industrial mass production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0036] A kind of vortioxetine hydrobromide tablet, its tablet core prescription is as follows:
[0037]
[0038]
Embodiment 2
[0040] A kind of vortioxetine hydrobromide tablet, its tablet core prescription is as follows:
[0041]
Embodiment 3
[0043] A kind of vortioxetine hydrobromide tablet, its tablet core prescription is as follows:
[0044]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
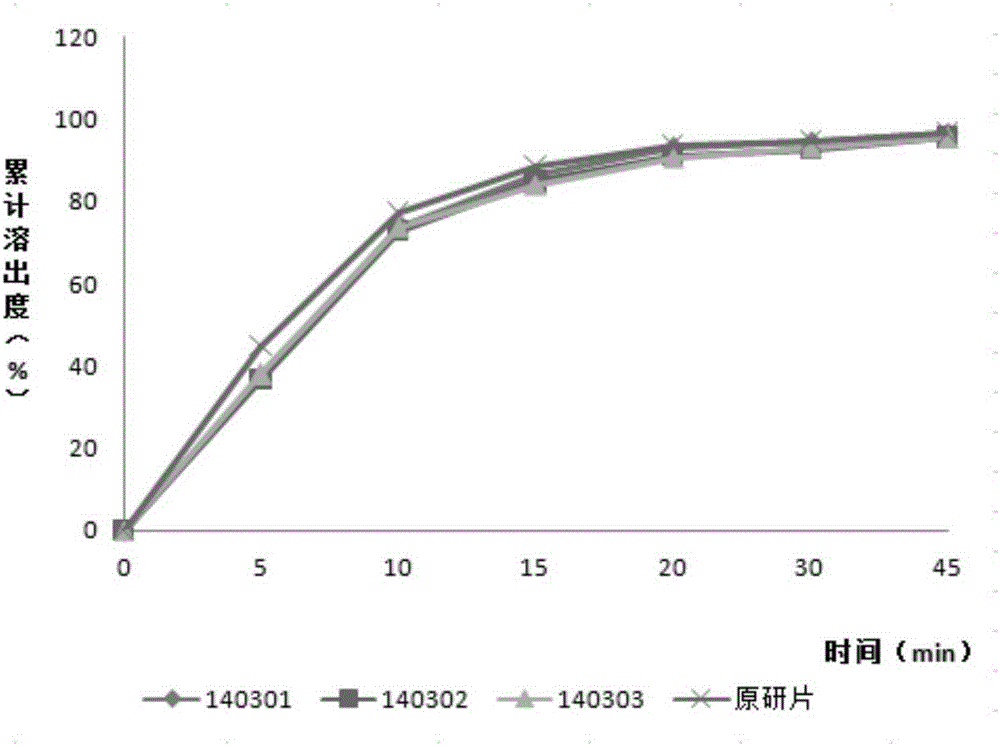
The invention relates to vortioxetine hydrobromide tablets and a preparation method thereof, and belongs to the technical field of medicine. Tablet cores of the vortioxetine hydrobromide tablets are prepared from vortioxetine hydrobromide, mannitol, calcium phosphate dibasic, sodium carboxymethyl starch and magnesium stearate. A coating solution of the vortioxetine hydrobromide tablets is prepared from hydroxypropyl methylcellulose, polyethylene glycol 400, titanium dioxide, iron oxide yellow and purified water. The preparation method includes the steps that the raw materials of the tablet cores are screened, evenly mixed, directly subjected to tabletting and then subjected to coating. After the tablets are subjected to gastric-soluble film coating, the bitter taste of the tablets can be well avoided when the tablets are taken orally, and therefore compliance is improved. A film coating layer contains titanium dioxide, shading can be well achieved, it is avoided that the tablets are degraded under illumination, and therefore the stability of the tablets is improved.
Description
technical field [0001] The invention relates to a vortioxetine hydrobromide tablet and a preparation method thereof, belonging to the technical field of medicine. Background technique [0002] Vortioxetine was jointly developed by Lundbeck and Takeda Pharmaceuticals in the United States. It was approved by the U.S. Food and Drug Administration (FDA) on September 30, 2013 to be marketed in the United States. and 20mg for the treatment of major depressive disorder in adults. [0003] Chinese Patent Publication No. CN104644594A discloses a vortioxetine hydrobromide gastric dissolving tablet and a preparation method thereof, the raw materials of which include vortioxetine hydrobromide, lubricant, filler, binder and disintegrant; The method includes steps such as granulation, and the granulation process takes a long time. In the process, the drug is exposed to light to easily degrade the raw material, which has a certain impact on the stability of the drug, and the production cy...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/36A61K31/495A61K47/02A61K47/36A61K47/26A61P25/24
Inventor 吴健民郑和校张静贺莲
Owner KAMP PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com