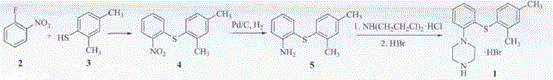Synthesis method suitable for industrialized production of vortioxetine hydrobromide
A kind of vortioxetine hydrobromide, suitable technology, applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, low yield, cumbersome operation, etc., and achieve the effect of simple operation, high yield and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
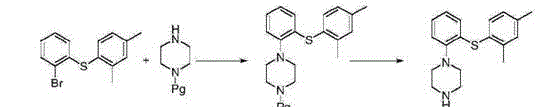
[0045] a. Preparation of Intermediate II:
[0046] 111g (1.0mol) of o-fluoroaniline and 327g (1.5mol) of Boc anhydride were stirred and refluxed in 2200ml of dry tetrahydrofuran for 48h, and the end point of the reaction was identified by TLC (developing solvent: dichloromethane-n-pentane=1:4), and the reaction was completed , cooled to room temperature, filtered, the filtrate was concentrated to dryness, the residue was added petroleum ether 1500ml, vigorously stirred for 30min, left to stand, the supernatant liquid was poured off, the residue was concentrated to dryness to obtain 205g colorless oil intermediate II, the yield 97.1%.
[0047] 1 H—NMR (500MHz, CDCl 3 / TMS,ppm):
[0048] δ 8.13 (m, 1H), 7.50 (m, 1H), 7.29 (m, 1H), 7.00 (bs, 1H), 6.89 (m, 1H), 1.54 (s, 9H).
[0049] b. Preparation of Intermediate III:
[0050]Intermediate II 190g (0.90mol), 2,4-dimethylthiophenol 125g (0.90mol) and DMF 1500ml, DIPEA (N,N-diisopropylethylamine) 149ml (0.91mol); at 85-90 Stir...
Embodiment 2
[0064] a. Preparation of Intermediate II:
[0065] 111g (1.0mol) of o-fluoroaniline and 327g (1.5mol) of Boc anhydride were stirred and refluxed in 2000ml of dry tetrahydrofuran for 48h, and the end point of the reaction was identified by TLC (developing solvent: dichloromethane-n-pentane=1:4), and the reaction was completed , cooled to room temperature, filtered, the filtrate was concentrated to dryness, the residue was added petroleum ether 2000ml, stirred vigorously for 30min, left to stand, the supernatant liquid was poured off, the residue was concentrated to dryness to obtain 201g colorless oil intermediate II, the yield 96.3%.
[0066] b. Preparation of Intermediate III:
[0067] Intermediate II 190g (0.90mol), 2,4-dimethylthiophenol 125g (0.90mol) and DMF 2000ml, DIPEA (N,N-diisopropylethylamine) 149ml (0.91mol); at 80-85 Stir at ℃ for 36 hours, TLC to identify the reaction end point (developing agent: dichloromethane-n-pentane = 1:3), after the reaction is completed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com