Method for determining cvortioxetine intermediate and its isomers by using liquid chromatography
A technology of isomers and determination methods, which is applied in the directions of measuring devices, material separation, and analysis materials, etc., can solve problems such as incomplete removal of isomers, side reactions, and influence on drug purity and quality, and achieve improvement Yield and purity, reduced side reactions, quality-controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Instruments and Conditions
[0038] LC-20AT pump, SPD-M20A detector, SIL-20AC autosampler, CBM-20A controller, CTO-10ASVP column thermostat, LC solution workstation
[0039] Chromatographic column: Welch (Ultimate PFP, 250×4.6mm, 5μm)
[0040] Mobile phase: A: 0.02mol / L dipotassium hydrogen phosphate buffer solution (adjust pH to 6.5 with phosphoric acid); B: methanol;
[0041] Elution was performed with the following gradient:
[0042]
[0043] Flow rate: 1.5mL / min
[0044] Detection wavelength: 220nm
[0045] Column temperature: 40°C
[0046] Injection volume: 10μL
[0047] Experimental procedure
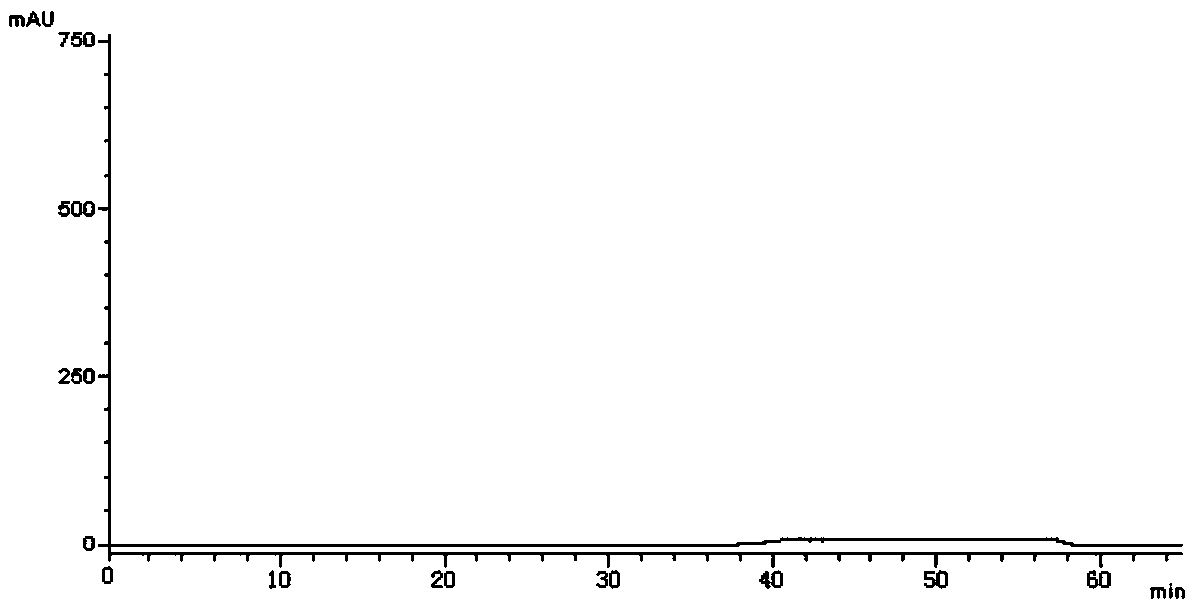
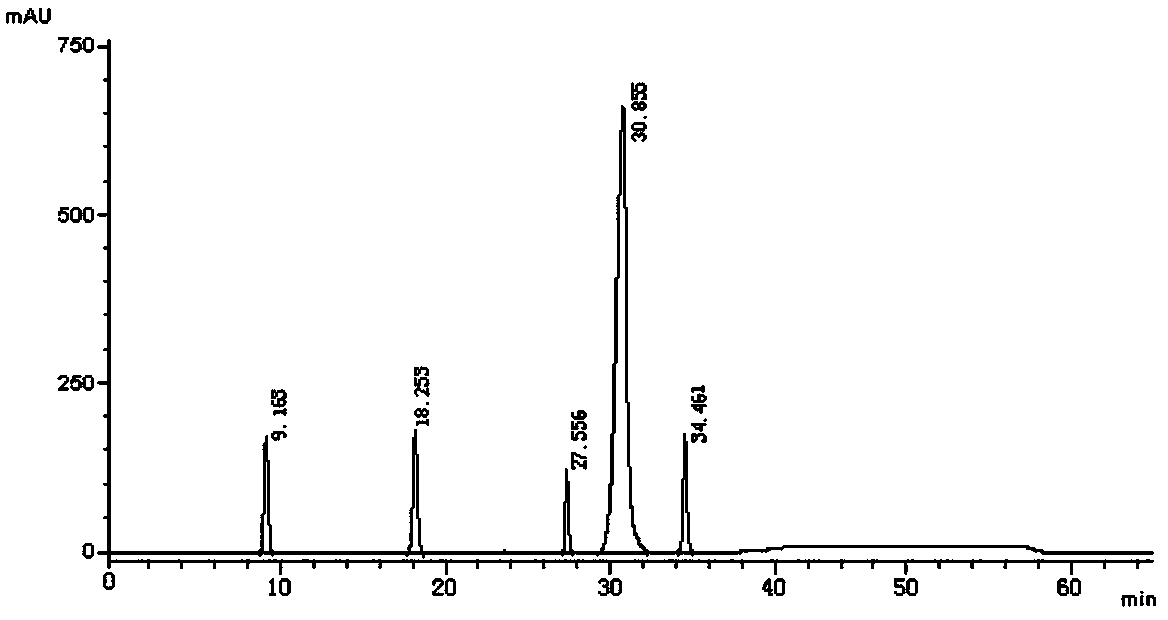
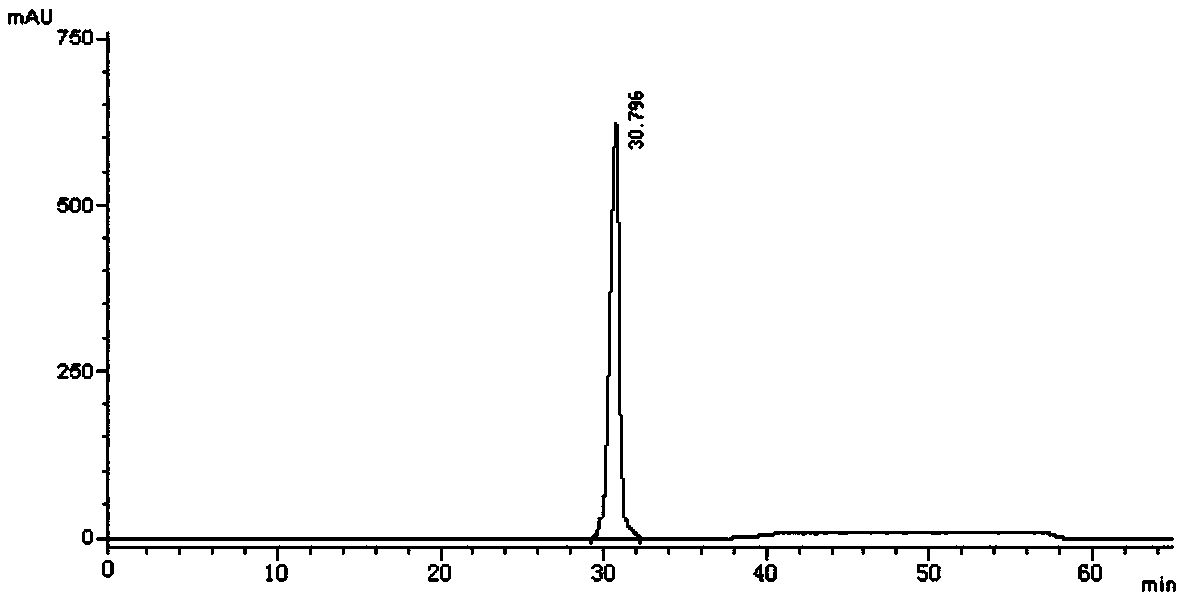
[0048] Take an appropriate amount of vortioxetine hydrobromide intermediate and its isomers, dissolve the sample with methanol, and prepare each 1mL containing about 0.5 mg of vortioxetine hydrobromide intermediate and its isomers sample solution. Take an appropriate amount of the above-mentioned vortioxetine hydrobromide intermediate and its isomer solution to p...
Embodiment 2
[0050] Instruments and Conditions
[0051] High performance liquid chromatography: Shimadzu: LC-20AT pump, SPD-M20A detector, SIL-20AC autosampler, CBM-20A controller, CTO-10ASVP column thermostat, LC solution workstation
[0052] Chromatographic column: Kromasil (100-5PHENYL, 250×4.6mm, 5μm)
[0053] Mobile phase: A: 1% triethylamine aqueous solution (adjust pH to 6.5 with phosphoric acid), B: methanol;
[0054] T(min) 0 15 35 53 53.01 65 B% 25 30 60 60 25 25
[0055] Flow rate: 1.5mL / min
[0056] Detection wavelength: 220nm
[0057] Column temperature: 40°C
[0058] Injection volume: 10μL
[0059] Experimental procedure
[0060] Take an appropriate amount of vortioxetine hydrobromide intermediate and its isomers, dissolve the sample with methanol, and prepare each 1mL containing about 0.5 mg of vortioxetine hydrobromide intermediate and its isomers sample solution. Take an appropriate amount of the above-mentioned vortioxetine hydrobromi...
Embodiment 3
[0062] Instruments and Conditions
[0063] High performance liquid chromatography: Shimadzu: LC-20AT pump, SPD-M20A detector, SIL-20AC autosampler, CBM-20A controller, CTO-10ASVP column thermostat, LC solution workstation
[0064] Chromatographic column: Agilent (ZORBAX SB-Phenyl, 250×4.6mm, 5μm)
[0065] Mobile phase: A: 0.05mol / L potassium dihydrogen phosphate buffer solution (adjust the pH to 6.5 with potassium hydroxide); B: methanol;
[0066] T(min) 0 15 35 55 55.01 65 B% 30 35 70 70 30 30
[0067] Flow rate: 1.5mL / min
[0068] Detection wavelength: 220nm
[0069] Column temperature: 40°C
[0070] Injection volume: 10μL
[0071] Experimental procedure
[0072] Take an appropriate amount of vortioxetine hydrobromide intermediate and its isomers, dissolve the sample with methanol, and prepare respectively about 0.5 mg of each 1 mL of vortioxetine hydrobromide intermediate and its isomers. sample solution. Take an appropriate amount of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com