Preparing method for vortioxetine hydrobromide alpha crystal form
A technology of vortioxetine hydrobromide and hydrobromic acid, applied in the direction of organic chemistry and the like, can solve the problems of large particle size, high desolvation temperature, low purity of vortioxetine hydrobromide α crystal form and the like , to achieve the effect of high crystal purity, low desolvation temperature and suitable particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
[0050] The preparation of embodiment 1-sec-butanol compound
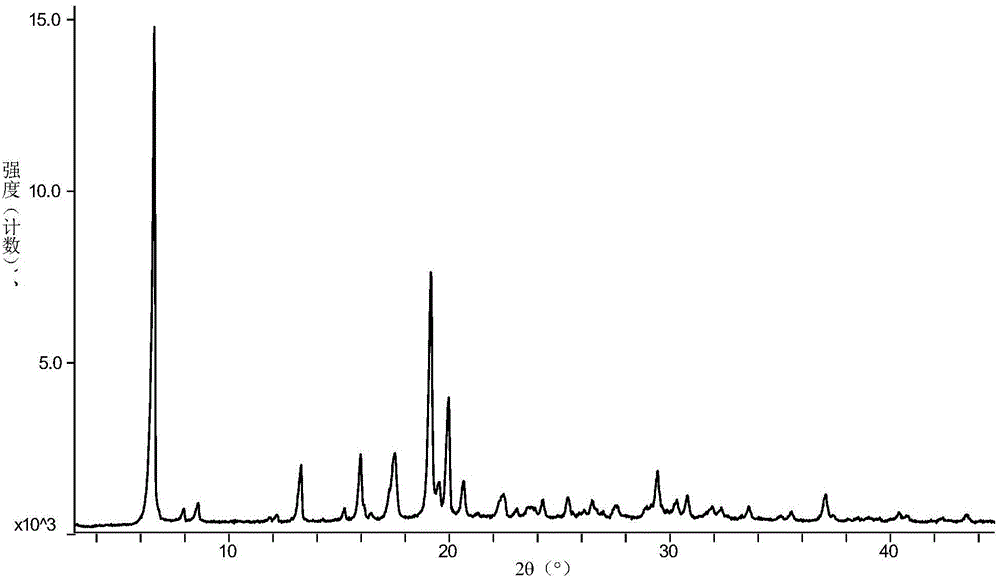
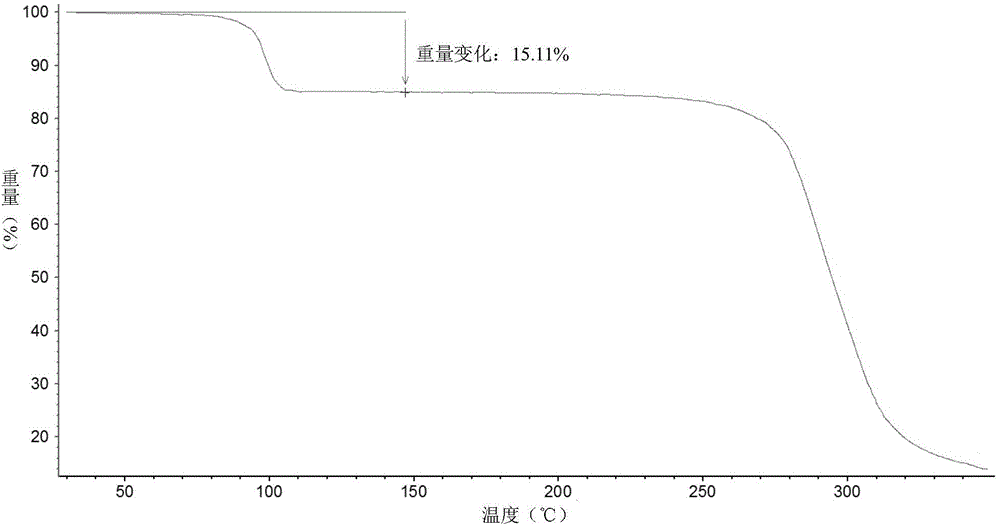
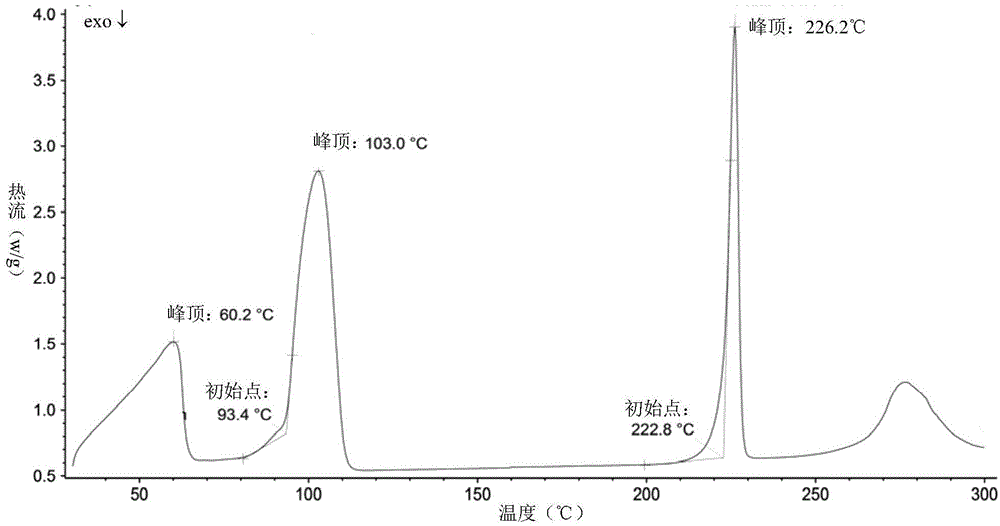
[0051] Add 29g of vortioxetine hydrobromide and 524ml of sec-butanol into a 1L reaction flask, heat to 100°C and reflux for 30min, then lower the temperature at a rate of 0.2°C / min until it is cooled to room temperature, filter, and dry at 30°C for 2h. 27.2 g of white solid was obtained. Its X-diffraction, TGA, DSC atlas see Figure 1~3 , and its X-ray powder diffraction data are shown in Table 2.
[0052] X-ray powder diffraction expressed in 2θ angles figure 1 There are characteristic peaks at 6.61±0.2, 13.26±0.2, 15.98±0.2, 17.47±0.2, 19.17±0.2, 19.97±0.2 and 20.64±0.2.
[0053] its TGA figure 2 The thermal weight loss is about 15.11%, which is close to one molecule of sec-butanol, so it is a sec-butanol compound.
[0054] Its DSC image 3 Among them, there are endothermic peaks at 60.2°C, 103.0°C and 226.2°C.
Embodiment 2 1
[0055] The preparation of embodiment 2-sec-butanol compound
[0056] Heat 5 g of vortioxetine hydrobromide and 100 ml of sec-butanol to 100°C and reflux for 30 minutes, then lower the temperature at a rate of 0.5°C / min until cooled to room temperature, filter, and dry at 20°C for 2 hours to obtain 4.8 g of white solid .
Embodiment 3 1
[0057] The preparation of embodiment 3-sec-butanol compound
[0058] Heat 5 g of vortioxetine hydrobromide and 100 ml of sec-butanol to 100°C and reflux for 30 minutes, then lower the temperature at a rate of 0.1°C / min until it cools to room temperature, filter, and dry at 40°C for 2 hours to obtain 4.5 g of white solid .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com