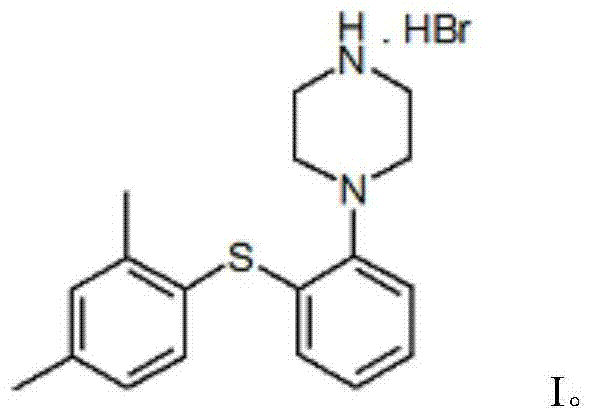
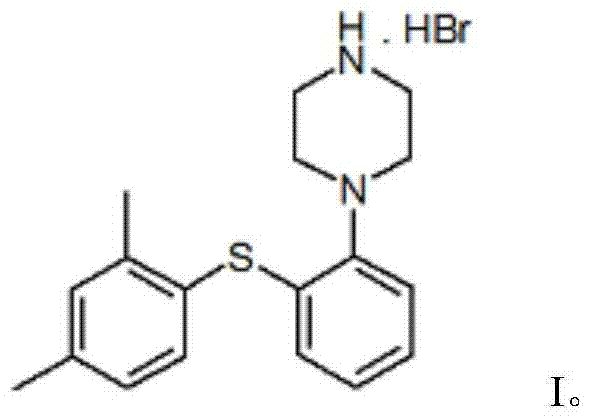
Vortioxetine hydrobromide
A technology of piperazine hydrobromide and dimethylphenylsulfanyl, used in the treatment of depression, especially the drug of severe depression in adults, the drug of 1-[2-phenyl]piperazine hydrobromide, Inhibitors of the serotonin transporter, the crystalline domain of the drug, can address issues such as SSRI blockage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Embodiment 1: Preparation of the crystallization (B crystal form) of the compound of formula I
[0241] (1) Dissolve 8 g of vortioxetine free base in toluene, add 1 equivalent of hydrobromic acid (48% hydrobromic acid aqueous solution), stir and crystallize, and filter the precipitate to obtain vortioxetine hydrobromic acid Salt (93%);
[0242] (2) Put 10 g of the product obtained in step (1) into a three-necked flask, add 100 g of toluene and 10 g of water, heat to 85 ° C and stir to dissolve, cool and crystallize, and dry the filtered crystals at 60-70 ° C to obtain hydrogen Vortioxetine bromate (90%);
[0243] (3) Put 1 g of the product obtained in step (2) into a three-necked flask, add 12 g of acetone, stir at 55-60° C. for 30 minutes to form a slurry, then stir at room temperature for 1.5 hours, filter, and vacuum-dry the obtained crystals to obtain It is a crystal of the B crystal form.
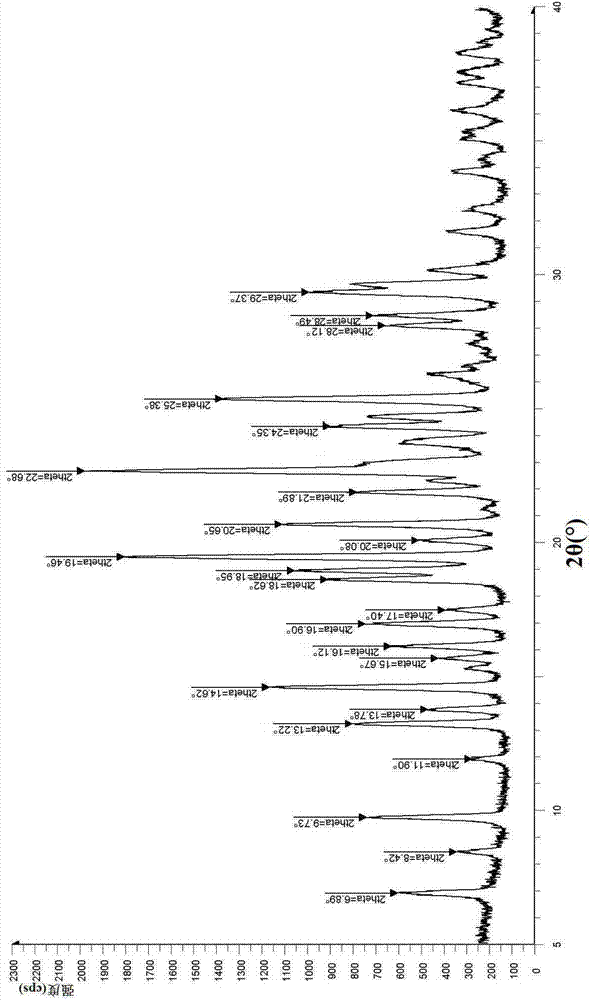
[0244] The powder X-ray diffraction figure of above-mentioned step (3)...
Embodiment 2
[0245] Embodiment 2: Preparation of the crystallization (B crystal form) of the compound of formula I
[0246] (1) Dissolve 8 g of vortioxetine free base in toluene, add 1.05 equivalents of hydrobromic acid (47% hydrobromic acid aqueous solution), stir and crystallize, and filter the precipitate to obtain vortioxetine hydrobromic acid Salt (91%);
[0247] (2) Put 10 g of the product obtained in step (1) into a three-necked flask, add 80 g of toluene and 8 g of water, heat to 85 ° C and stir to dissolve, cool and crystallize, and dry the filtered crystals at a temperature of 60-70 ° C to obtain hydrogen Vortioxetine bromate (92%);
[0248] (3) Put 1 g of the product obtained in step (2) into a three-necked flask, add 10 g of acetone, stir at 55-60°C for 20 minutes to form a slurry, then stir at room temperature for 1 hour, filter, and vacuum-dry the obtained crystals to obtain It is a crystal of the B crystal form.
Embodiment 3
[0250] Embodiment 3: Preparation of the crystallization (B crystal form) of the compound of formula I
[0251] (1) Dissolve 8 g of vortioxetine free base in toluene, add 0.95 equivalent of hydrobromic acid (47% hydrobromic acid aqueous solution), stir and crystallize, and filter the precipitate to obtain vortioxetine hydrobromic acid Salt (92%);
[0252] (2) Put 10 g of the product obtained in step (1) into a three-necked flask, add 120 g of toluene and 12 g of water, heat to 85 ° C and stir to dissolve, cool and crystallize, and dry the filtered crystals at 60-70 ° C to obtain hydrogen Vortioxetine bromate (91%);
[0253] (3) Put 1 g of the product obtained in step (2) into a three-necked flask, add 15 g of acetone, stir at 55-60° C. for 40 minutes to form a slurry, then stir at room temperature for 2 hours, filter, and vacuum-dry the obtained crystals to obtain It is a crystal of the B crystal form.
[0254] The powder X-ray diffraction figure of above-mentioned step (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com