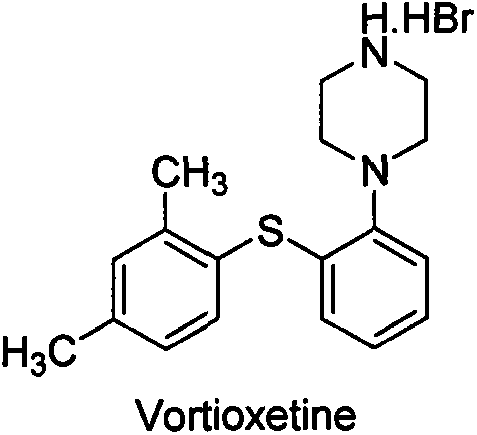
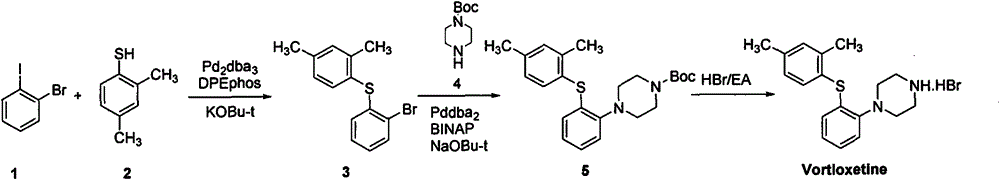
Refining and crystal transformation method for vortioxetine hydrobromide
A technology of vortioxetine hydrobromide and crystal transformation, applied in organic chemistry methods, organic chemistry, etc., can solve problems such as inorganic salt residues, toluene solvent residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: (repeat the embodiment of the original research CN102617513A)
[0024] 100g of crude tioxetine hydrobromide was dissolved in a mixed solvent (10:1, V / V) of toluene (800mL) and water (80mL) at 80°C, and then filtered hot; the filtrate was cooled to 9°C, crystallized, Filtration; the filter cake was washed with toluene (50mLx2), dried in vacuum at 50°C to constant weight, and 75g of white crystals were obtained, with a yield of 75%, an HPLC purity of 99.83%, a maximum single impurity of less than 0.1%, and a total impurity of less than 0.17%; the residue> 0.1% is unqualified; GC detection of toluene>0.15% is unqualified; XRD is consistent with the β crystal form data of vortioxetine hydrobromide reported by the original CN102617513A.
Embodiment 2
[0026] Use 200mL of methyl tert-butyl ether and ethanol mixed solvent (10:1, V / V) to beat 100g of tioxetine crude product at 50°C for 1 hour, filter; Slurry for 1 hour, filter; filter cake is heated to reflux with 400mL n-butanol and water mixed solvent (10:1, V / V) to dissolve, heat filter, cool to 9°C, crystallize, filter; filter cake is mixed with 300mL water After boiling, 150mL of water was distilled off, and the solution was slowly cooled to 9°C. After standing for 2 hours, crystallized and filtered, the filter cake was washed with water (50mLx2), and dried in vacuum at 50°C to constant weight to obtain 65g of white crystals. The yield 65%, HPLC purity 99.83%, maximum single impurity no more than 0.1%, total impurity no more than 0.17%; residue <0.01% qualified; GC detection of n-butanol not detected; XRD and original research CN102617513A reported vortioxetine hydrobromide The β crystal form data are consistent.
Embodiment 3
[0028] 100g of crude vortioxetine hydrobromide was beaten with 200mL of methyl tert-butyl ether and n-butanol mixed solvent (10:1, V / V) at 50°C for 1 hour, and filtered; Beat the slurry for 1 hour, filter; the filter cake is heated to reflux with 400mL n-butanol and water mixed solvent (10:1, V / V) to dissolve, heat filter, cool to 9 ° C, crystallize, filter; filter cake in 300mL water After azeotropy, 150mL of water was distilled off, and the solution was slowly cooled to 9°C. After standing for 2 hours, crystallized and filtered, the filter cake was washed with water (50mLx2), and dried in vacuum at 50°C to constant weight to obtain 74g of white crystals. The yield is 74%, the HPLC purity is 99.93%, the maximum single impurity is no more than 0.03%, the total impurity is no more than 0.07%; the residue <0.01% is qualified; GC detection of n-butanol is not detected; The data of Ting β crystal form are consistent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com