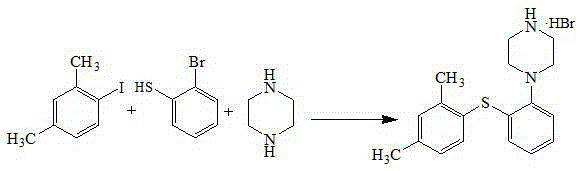
Synthetic method for vortioxetine hydrobromide
A technology for the synthesis of vortioxetine hydrobromide and its synthesis method, which is applied in the field of synthesis of vortioxetine hydrobromide, can solve the problems of harsh reaction conditions, low product purity, and high price, and achieve mild reaction conditions and high purity , the effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
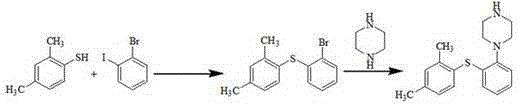
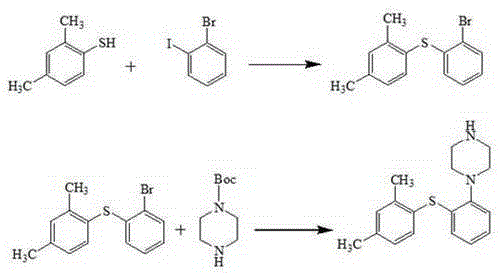
[0037] The synthesis of embodiment 12-(2,4-dimethylphenylsulfanyl) nitrobenzene
[0038] Add o-nitrothiophenol (14.5g, 0.1mol), 2,4-dimethyliodobenzene (25.5g, 1.1eq) and 145mL tetrahydrofuran into a dry 250mL four-necked reaction flask, and add sodium hydride ( 3.6g, 1.5eq), react at room temperature for 2 hours. After the reaction was completed, the solvent was removed by rotary evaporation, 145 mL of toluene and 145 mL of water were added, the liquid was extracted and separated, the toluene layer was dried by adding anhydrous sodium sulfate, decolorized by activated carbon, and then the solvent was evaporated to obtain a yellow-brown oily substance 2-(2,4-dimethyl phenylthio) nitrobenzene 23.2g, yield 89.6%, purity 99.5%. MS(m / z):260[M+H] + ; 1 HNMR (400MHz, CDCl 3 )δ: 8.24(dd, J=8.2, 1.3Hz, 1H), 7.47(d, J=7.8Hz, 1H), 7.33~7.28(m, 1H), 7.22~7.15(m, 2H), 7.11(d ,J=7.8Hz,1H),6.72(dd,J=8.2,1.1Hz,1H),2.40(s,3H),2.31(s,3H).
Embodiment 2
[0039] The synthesis of embodiment 22-(2,4-dimethylphenylsulfanyl) nitrobenzene
[0040] Add o-nitrothiophenol (14.5g, 0.1mol), 2,4-dimethylbromobenzene (20.4g, 1.1eq) and tetrahydrofuran 145mL into a dry 250mL four-necked reaction flask, and add sodium hydride ( 3.6g, 1.5eq), react at room temperature for 2 hours. After the reaction was completed, the solvent was removed by rotary evaporation, 145 mL of toluene and 145 mL of water were added, the liquid was extracted and separated, the toluene layer was dried by adding anhydrous sodium sulfate, decolorized by activated carbon, and then the solvent was evaporated to obtain a yellow-brown oily substance 2-(2,4-dimethyl phenylthio) nitrobenzene 20.8g, yield 80.3%, purity 99.4%.
Embodiment 3
[0041] Synthesis of Example 32-(2,4-dimethylphenylthio)nitrobenzene
[0042] Add o-nitrothiophenol (14.5g, 0.1mol), 2,4-dimethyliodobenzene (25.5g, 1.1eq) and tetrahydrofuran 145mL into a dry 250mL four-necked reaction flask, and add calcium hydride ( 6.15g, 1.5eq), react at room temperature for 2 hours. After the reaction was completed, the solvent was removed by rotary evaporation, 145 mL of toluene and 145 mL of water were added, the liquid was extracted and separated, the toluene layer was dried by adding anhydrous sodium sulfate, decolorized by activated carbon, and then the solvent was evaporated to obtain a yellow-brown oily substance 2-(2,4-dimethyl phenylthio) nitrobenzene 21.3g, yield 82.1%, purity 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com