Crystal form of vortioxetine hydrobromide and preparation method thereof
A technology for vortioxetine crystals and vortioxetine, which is applied in the field of medicine, can solve problems such as solvent residues and the like, and achieve the effects of complete crystallization, short preparation time and low usage of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
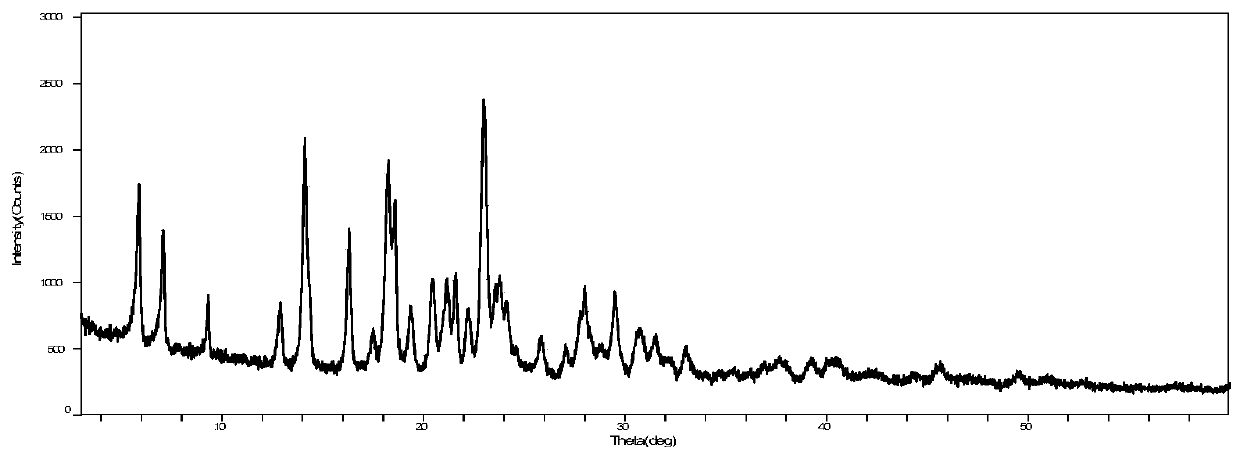
[0053] Take 5 g of vortioxetine hydrobromide and 50 ml of sec-butanol, and add them to a 100 ml round bottom flask. Raise the temperature to 80°C, if it is not dissolved, add 5ml of water, dissolve it, and maintain it for 0.5h. The temperature was lowered, and a large amount of solids were precipitated at 15°C, and the temperature was continued to drop to 0°C. Insulate and grow the crystal for 2h. Suction filtration to obtain a white solid. It was air-dried at 90°C for 2 hours and weighed 4.33g. Yield 86.6%, purity 99.9%. Its XRPD pattern and figure 1 be consistent.
Embodiment 2
[0055] Take 5 g of vortioxetine hydrobromide and 50 ml of sec-butanol, and add them to a 100 ml round bottom flask. Raise the temperature to 81°C, if it is not dissolved, add 2.5ml of water, dissolve it, and maintain it for 0.5h. When the temperature was lowered to 45°C, a large amount of solids were precipitated, and the temperature was continued to drop to 0°C. Insulate and grow the crystal for 2h. Suction filtration to obtain a white solid. Air-dried at 90°C for 2 hours, weighing 4.73g. Yield 94.6%, purity 99.9%. Its XRPD pattern and figure 1 be consistent.
Embodiment 3
[0057] Take 5 g of vortioxetine hydrobromide and 50 ml of sec-butanol, and add them to a 100 ml round bottom flask. Raise the temperature to 82°C, if it is not dissolved, add 1.7ml of water, dissolve it, and maintain it for 0.5h. When the temperature was lowered to 47°C, a large amount of solids were precipitated, and the temperature was continued to drop to 0°C. Insulate and grow the crystal for 2h. Suction filtration to obtain a white solid. It was air-dried at 90°C for 2 hours and weighed 4.78g. Yield 95.6%, purity 99.9%. Its XRPD pattern and figure 1 be consistent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com