Preparation method of vortioxetine hydrobromide impurity
A technology of vortioxetine hydrobromide and impurities, applied in directions such as organic chemistry, can solve problems such as few synthetic reports, and achieve the effects of cheap raw materials, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Mix piperazine, o-bromoiodobenzene, sodium tert-butoxide, bis(dibenzylideneacetone) palladium, and toluene evenly, replace the air with nitrogen, reflux for 6.5 hours, cool and filter, wash the filtrate with aqueous sodium chloride, and dry , concentrated to dryness, and then obtained 2-bromophenylpiperazine by column chromatography, wherein the molar ratio of piperazine to o-bromoiodobenzene is 1:1.5, and the molar ratio of piperazine to sodium tert-butoxide is 1:3 , the molar ratio of piperazine and bis(dibenzylideneacetone) palladium is 1:0.005, the weight volume (g / ml) ratio of piperazine and toluene is 1:20, and the column chromatography mobile phase is dichloromethane and methanol Mixed solution, wherein, the volume ratio of dichloromethane and methanol is 20:1;
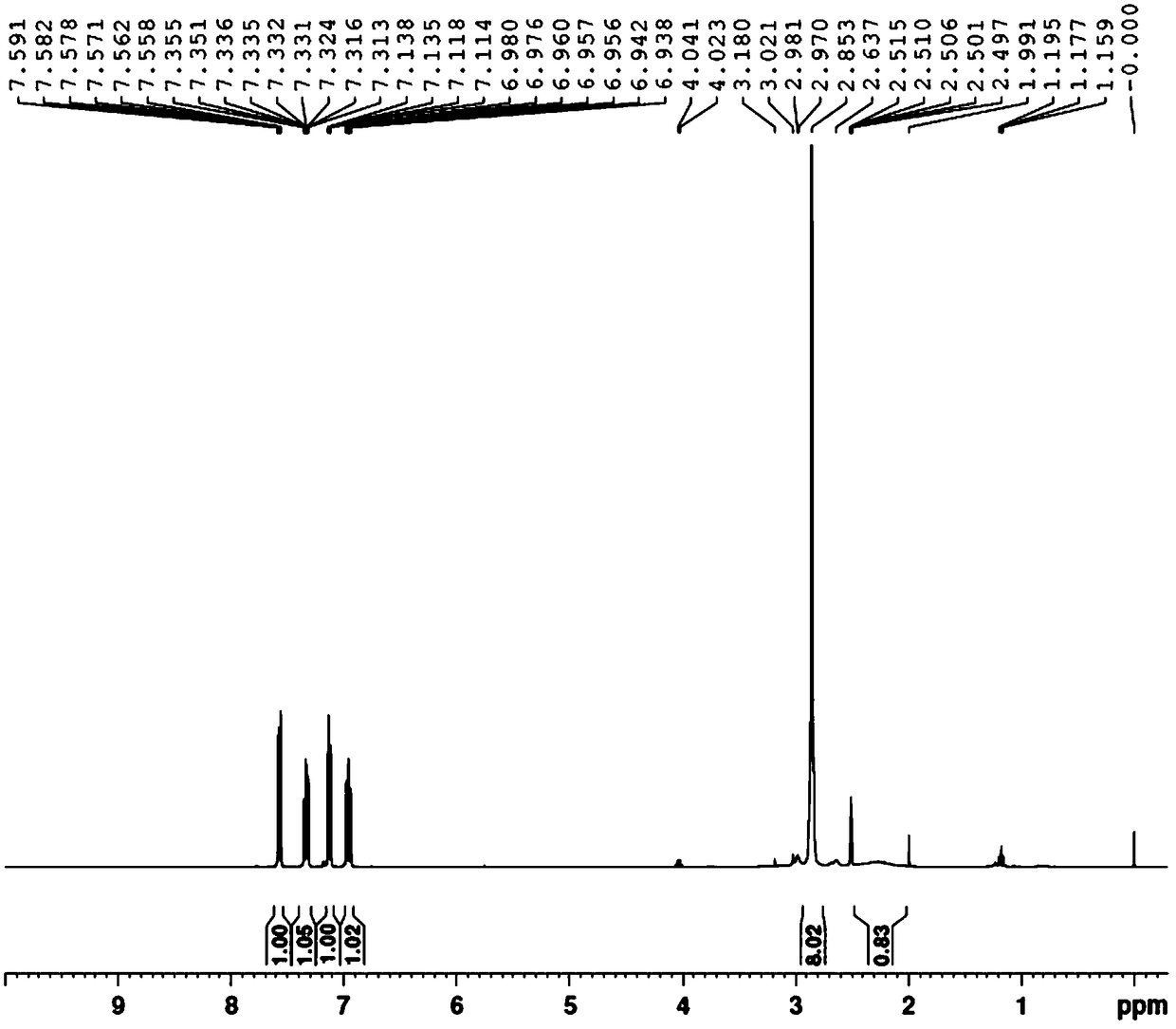
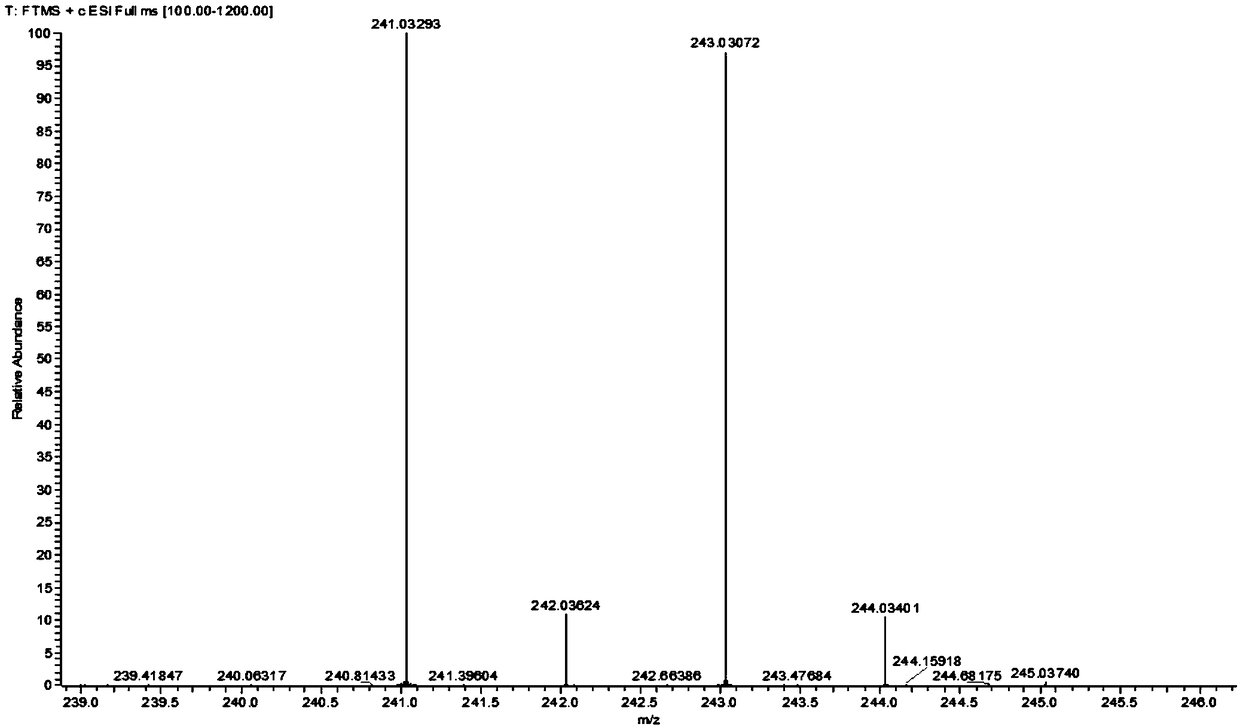
[0034] The proton nuclear magnetic resonance spectrum, mass spectrogram of gained 2-bromophenylpiperazine see figure 1 , figure 2 .
Embodiment 2
[0036] Mix piperazine, o-bromoiodobenzene, sodium tert-butoxide, bis(dibenzylideneacetone) palladium, and toluene evenly, replace the air with nitrogen, reflux for 6.5 hours, cool and filter, wash the filtrate with aqueous sodium chloride, and dry , concentrated to dryness, and then obtained 1,4-bis(2-bromophenyl)piperazine by column chromatography, wherein the molar ratio of piperazine to o-bromoiodobenzene was 1:2, piperazine, sodium tert-butoxide The molar ratio of piperazine and bis(dibenzylideneacetone) palladium is 1:3, the molar ratio of piperazine and bis(dibenzylideneacetone)palladium is 1:0.005, the weight volume (g / ml) ratio of piperazine and toluene is 1:20, and the column chromatography flow The phase is a mixed solution of petroleum ether and ethyl acetate, wherein the volume ratio of petroleum ether and ethyl acetate is 100:1;
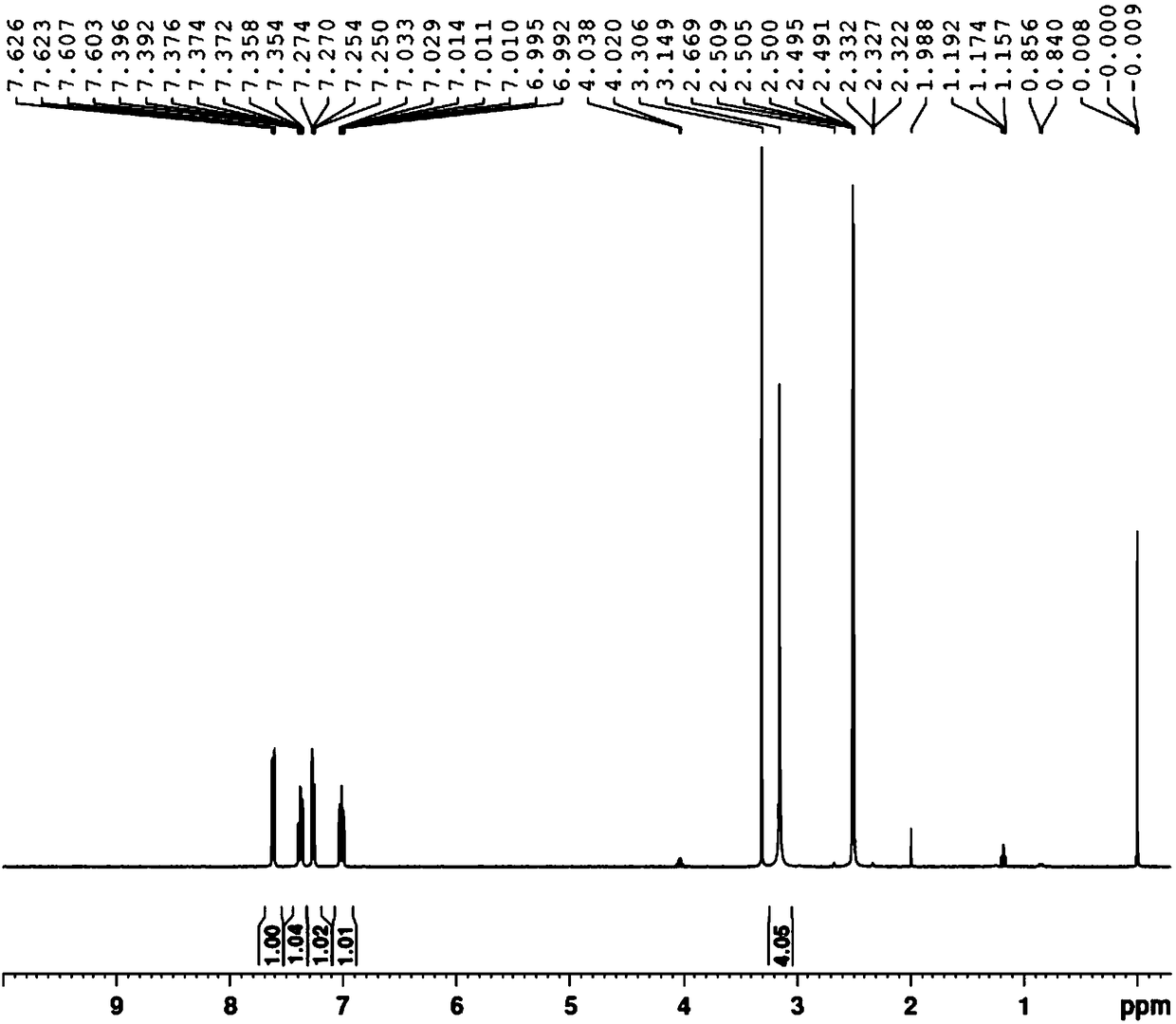
[0037] Gained 1,4-two (2-bromophenyl) piperazine proton nuclear magnetic resonance spectrogram, mass spectrogram see image 3 , Figu...
Embodiment 3
[0039] Mix piperazine, o-bromoiodobenzene, triethylamine, bis(dibenzylideneacetone) palladium, and toluene, replace the air with nitrogen, reflux for 5 hours, cool and filter, wash the filtrate with aqueous sodium chloride, dry, and concentrate to dryness, and then obtain 2-bromophenylpiperazine by column chromatography, wherein the molar ratio of piperazine to o-bromoiodobenzene is 1:1, the molar ratio of piperazine and triethylamine is 1:5, and piperazine , The molar ratio of bis(dibenzylideneacetone) palladium is 1:0.003, the weight volume (g / ml) ratio of piperazine and toluene is 1:15, and the column chromatography mobile phase is a mixed solution of dichloromethane and methanol, Wherein, the volume ratio of dichloromethane and methanol is 30:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com