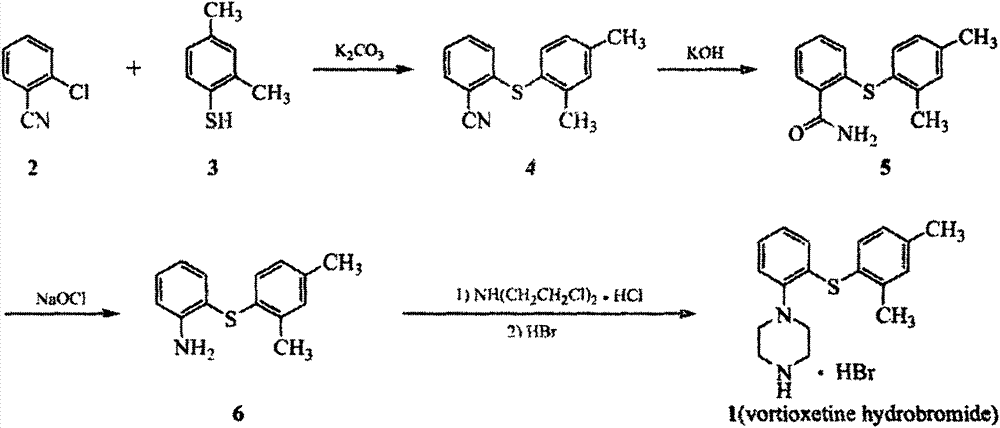
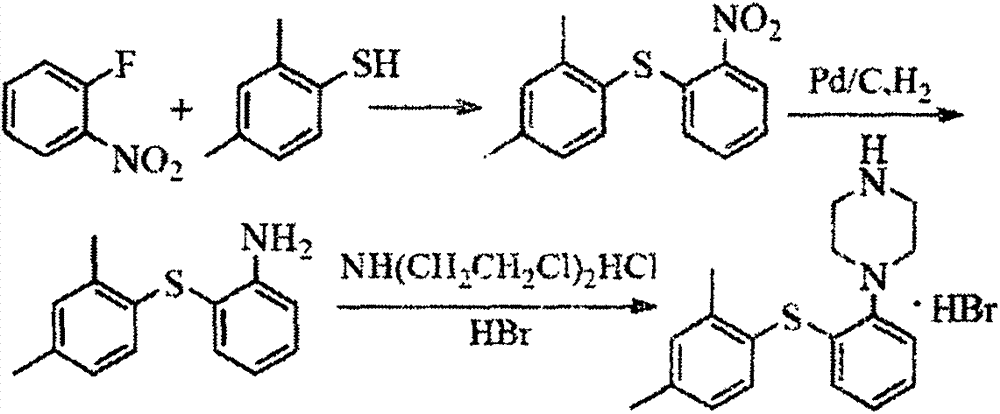
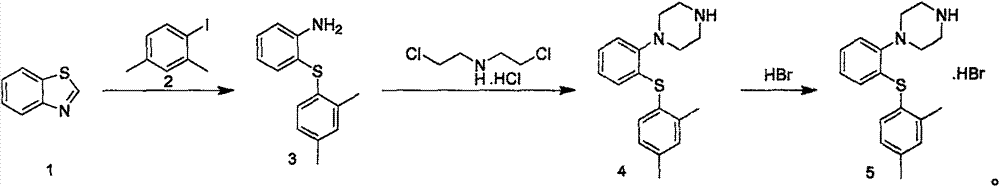
New synthesis process for vortioxetine hydrobromide
A technology of vortioxetine hydrobromide and a synthetic method, which is applied in the field of medicine and chemical industry, can solve the problems of high equipment requirements, low yield, and increased cost, and achieve short method routes, easily available raw materials, and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1. compound 3:
[0022] Under nitrogen atmosphere, the FeCl 3 (3.2g, 0.2eq), trans-1,2 cyclohexanediamine (5.6g, 0.4eq), benzothiazole (13.5g), 2,4-dimethyliodobenzene (34.5g) NaOH (12g, 3eq), and 100mL of water were placed in a 250mL round-bottomed flask and reacted at 110°C for 4 hours. After the reaction was completed, it was extracted with ethyl acetate, dried, and separated by column chromatography to obtain 18g of the target product with a yield of 78.3%.
Embodiment 2
[0023] Embodiment 2, the synthesis of compound 4:
[0024] Compound 4 (18g, 78mmol) was dissolved in 150mL of acetonitrile, added dichloroethylamine hydrochloride (14.3g, 80mmol), and DIEA (32g, 4eq), refluxed for 4 hours, concentrated, diluted with water, ethyl acetate Extraction, drying, and the resulting crude product were used directly in the next step without purification.
Embodiment 3
[0025] Embodiment 3, the synthesis of compound 5:
[0026] The crude product obtained in the previous step was redissolved in 70 ml of ethanol, and 23 g of HBr aqueous solution with a mass fraction of 40% was added under stirring, heated until the solution was clear, then gradually cooled to 0° C., a white solid was precipitated, filtered , the filter cake was washed with cold ethanol solution (15mL*2) and dried to obtain a white solid 18.4g, yield 61%, purity 98.5%, mp 231.1-232.3°C. MS(m / z): 299[M+H] + ; 1 H NMR (400MHz, CDCl 3 +D 2 O): 7.32(d, J=7.8Hz, 1H), 7.15(s, 1H), 7.09(d, J=3.9Hz, 2H), 7.03(d, J=7.2Hz, 1H), 6.99~6.85( m, 1H), 6.54 (d, J=7.8Hz, 1H), 3.46 (dd, J=15.8, 5.4Hz, 8H). 2.33 (d, J=24.5Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com