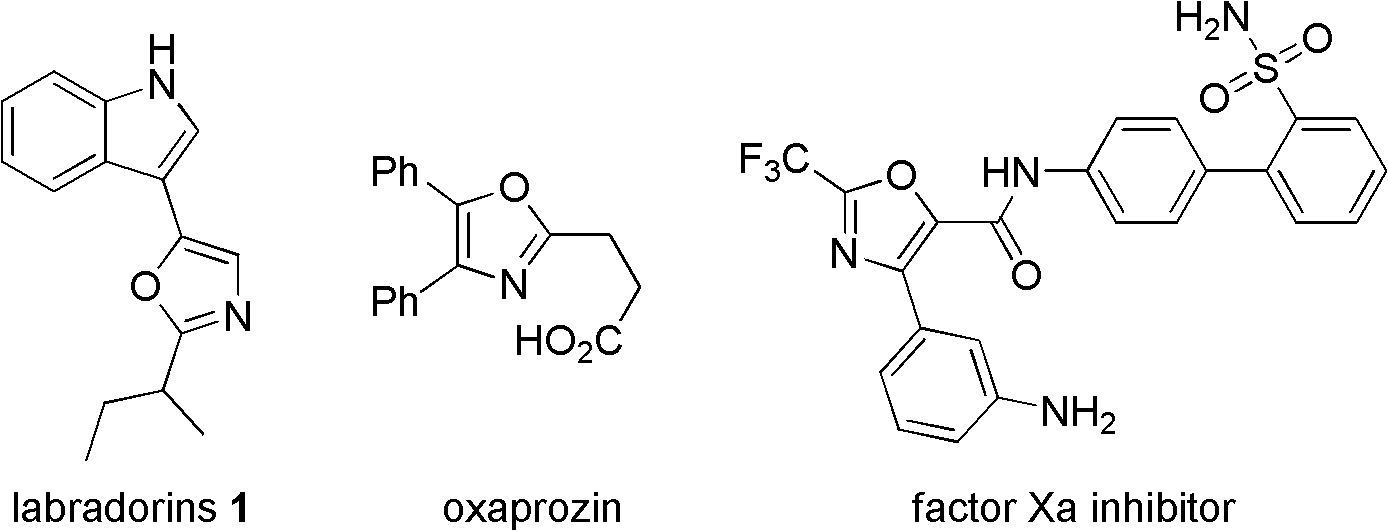
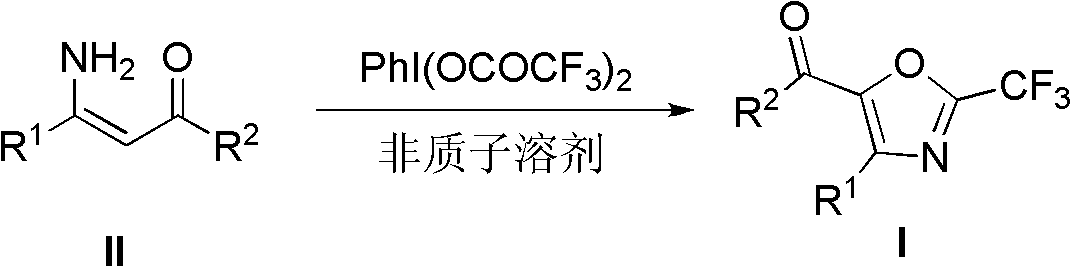
Synthesis method of 2-trifluoromethyloxazole derivatives
A technology for trifluoromethyl oxazoles and a synthesis method, applied in the field of synthesis of 2-trifluoromethyl oxazole derivatives, can solve the problems of difficult preparation of raw materials, high synthesis cost, single substitution type, etc. Gentle, easy-to-use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
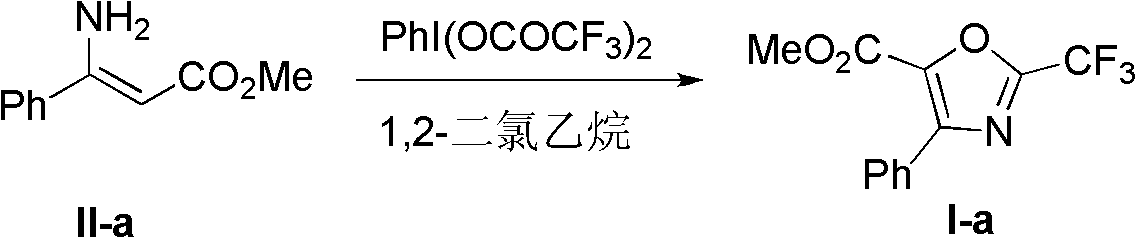
[0031] Preparation of 2-trifluoromethyl-4-phenyl-5-methoxycarbonyloxazole (I-a):
[0032]
[0033] Bis(trifluoroacetoxy)iodobenzene (0.243 g, 0.0006 mol) was dissolved in dry 1,2-dichloroethane (6 mL), stirred and heated to 40°C. Dissolve methyl 3-amino-3-phenylacrylate (II-a) (0.1g, 0.0006mol) in dry 1,2-dichloroethane (12mL) and add dropwise to the above solution within 8 minutes , stirred at 45°C for 1 h until the reaction was complete, the solution was added to silica gel (0.12 g) and evaporated to dryness, separated by column chromatography (ethyl acetate: petroleum ether = 1:99) to obtain 0.104 g of white crystals, with a yield of 73%. Melting point: 47-48°C.
[0034] 1 H-NMR (CDCl 3 , 400MHz) δ: 8.11-8.12 (m, 2H), 7.50-7.51 (m, 3H), 4.00 (s, 3H). 13 C-NMR (CDCl 3 , 100MHz) δ: 157.93(C), 150.07~150.40(q, J C-F =45Hz, C), 146.85(C), 137.83(C), 130.52(C), 129.36(CH), 128.49(CH), 128.40(CH), 114.71~117.41(q, J C-F =270Hz, CF 3 ), 52.76 (OCH 3 ). 19 F-NMR (CDCl ...
Embodiment 2
[0036] Preparation of 2-trifluoromethyl-4-p-bromophenyl-5-methoxycarbonyloxazole (I-b):
[0037]
[0038]Bis(trifluoroacetoxy)iodobenzene (0.174 g, 0.0004 mol) was dissolved in dry anhydrous dichloromethane (4 mL), stirred at 40°C until constant temperature. Dissolve 3-amino-3-p-bromophenylmethylacrylate (II-b) (0.1g, 0.0004mol) in dry anhydrous dichloroethane (8mL) and add dropwise to the above solution within 8 minutes , stirred at 50°C for 1.5h until the reaction was complete, the solution was added to silica gel (0.12g) and evaporated to dryness, separated by column chromatography (ethyl acetate:petroleum ether=1:99) to obtain 0.078g of white solid, yield 58%. Melting point: 74-75°C.
[0039] 1 H-NMR (CDCl 3 , 400MHz) δ: 8.03~8.05 (d, 2H, J=8.4Hz), 7.60~7.63 (d, 2H, J=8.8Hz), 4.00 (s, 3H). 13 C-NMR (CDCl 3 , 100MHz) δ: 157.84(C), 150.52~150.96(q, J C-F =45Hz, C), 145.79(C), 137.93(C), 131.67(C), 130.87(CH), 127.36(CH), 125.18(C), 114.61~117.32(q, J C-F =270Hz, CF...
Embodiment 3
[0041] Preparation of 2-trifluoromethyl-4-o-methoxyphenyl-5-methoxycarbonyloxazole (I-c):
[0042]
[0043] Bis(trifluoroacetoxy)iodobenzene (0.229 g, 0.0005 mol) was dissolved in dry 1,2-dichloroethane (5 mL), stirred at 60°C until constant temperature. Dissolve methyl 3-amino-3-o-methoxyphenylacrylate (II-c) (0.1 g, 0.0005 mol) in dry 1,2-dichloroethane (10 mL) and drop it over 8 minutes Add the above solution, stir at 45°C for 1 h until the reaction is complete, add silica gel (0.12 g) to the solution and evaporate to dryness, and separate by column chromatography (ethyl acetate:petroleum ether=1:99) to obtain 0.084 g of a lavender solid, yield 60%. Melting point: 48-49°C.
[0044] 1 H-NMR (CDCl 3 , 400MHz) δ: 7.51~7.53(dd, 1H, J=1.6Hz, J=7.6Hz), 7.45~7.49(m, 1H), 7.04~7.10(t, 1H, J=7.2Hz), 7.00~7.02 (d, 1H, J=8.4Hz), 3.89(s, 3H), 3.82(s, 3H). 13 C-NMR (CDCl 3 , 100MHz) δ: 157.75(C), 157.16(C), 150.44~150.91(d, J C-F =47Hz, C), 142.66(C), 140.08(CH), 131.58(C), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com