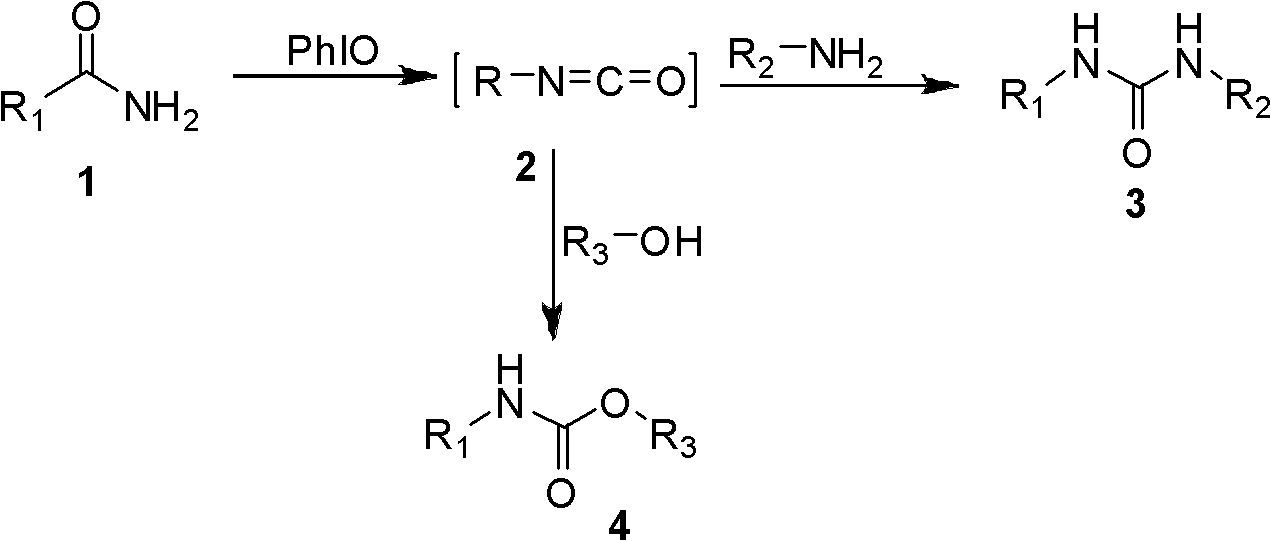
Synthetic method of 1, 3-two substituted ureas and carbamate
A technology of carbamate and disubstituted urea, applied in 1 field, can solve problems such as difficult preparation of asymmetric urea, complex experimental operation, strong toxicity of raw materials, etc., and achieve cheap raw materials, simple experimental operation, and easy-to-use products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, preparation of symmetrical 1,3-disubstituted urea
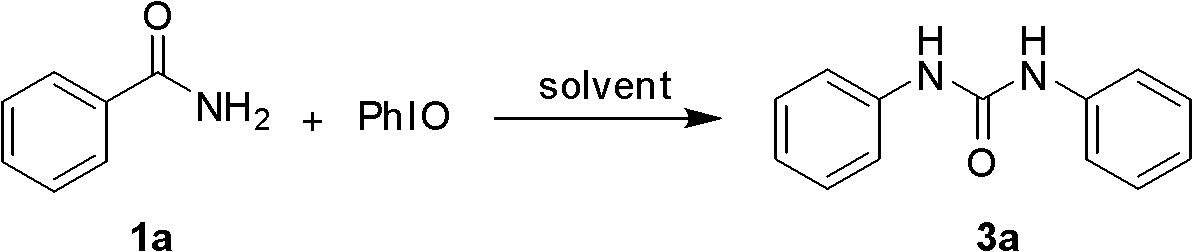
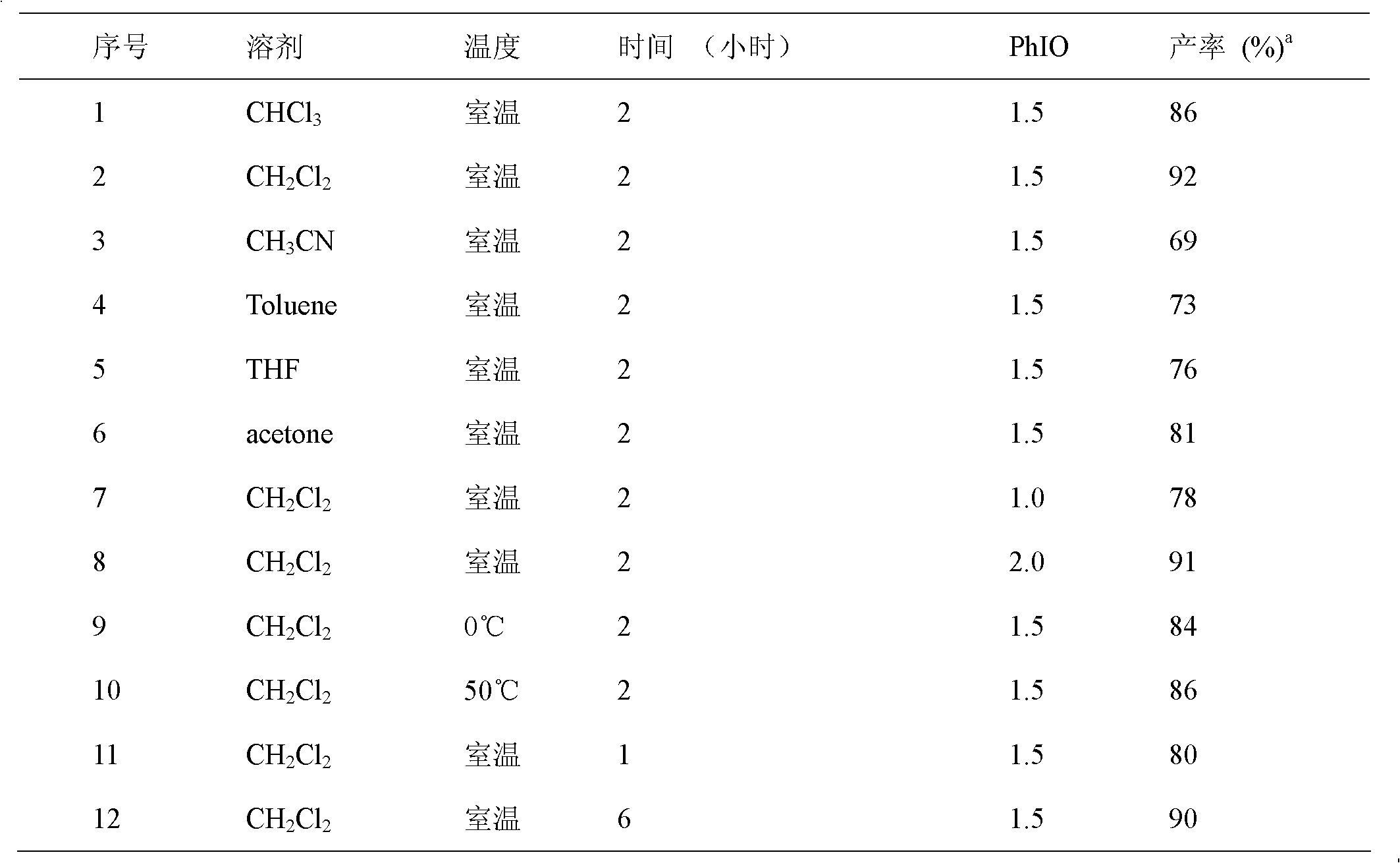
[0020] Screen the reaction conditions for the preparation of symmetrical 1,3-disubstituted urea 2a, including the reaction solvent, the amount of hypervalent iodine, the reaction temperature, and the reaction time. It was found that using dichloromethane as a solvent, adding iodobenzene oxide in a molar ratio of 1.5 times, and reacting at room temperature for 2 hours, the best yield of urea was 92%. The following preparations of urea are based on this standard condition.
[0021] Table 1: Screening of reaction conditions.
[0022]
[0023]
[0024] a Isolated yield
[0025] Dissolve the amide in dichloromethane, add iodobenzene oxide at a molar ratio of 1.5 times, and stir the reaction solution at room temperature for 2 hours. The reaction solution was filtered, concentrated, and petroleum ether and ethyl acetate were used as eluents, and the symmetrical 1,3-disubstituted urea was obtained by co...
Embodiment 2
[0032] Embodiment 2, preparation of asymmetric 1,3-disubstituted urea
[0033] The amide was dissolved in dichloromethane, 1.5 times molar ratio of amine and iodobenzene oxide were sequentially added, and the reaction solution was stirred at room temperature for 2 hours. The reaction solution was filtered and concentrated. The unsymmetrical 1,3-disubstituted urea was obtained by column chromatography with petroleum ether and ethyl acetate as eluent. The asymmetric 1,3-disubstituted urea prepared by this method and its yield are shown in Table 2, and all products have been proved by H NMR spectrum. Amides in this method include: aryl amides and aliphatic amides. No matter it is linear amine or cyclic amine, 1,3-disubstituted urea can be prepared in high yield. When diisopropylamine with large steric hindrance is used as the nucleophile, the yield of the prepared 1,3-disubstituted urea is as high as 93%.
[0034] Table 2: Preparation of unsymmetrical 1,3-disubstituted urea ...
Embodiment 3
[0057] Embodiment three, prepare carbamate
[0058] The amide was dissolved in alcohol, and 1.5 times molar ratio of iodobenzene oxide was added, and the reaction solution was stirred at room temperature for 2 hours. The reaction solution was filtered and concentrated. Carbamate was obtained by column chromatography using petroleum ether and ethyl acetate as eluent. The carbamates prepared by this method and their yields are shown in Table 3, and all products have been proved by proton nuclear magnetic resonance. Amines useful in this method include aryl amines as well as aliphatic amines. The alcohol used in this method has methanol. When isopropanol or tert-butanol with relatively large steric hindrance is used as nucleophile, no carbamate is formed and only symmetrical urea is obtained. The alkynyl group has no effect on the reaction when propargyl alcohol is used as the nucleophile.
[0059] Table 3: Preparation of carbamates
[0060]
[0061]
[0062] Reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com