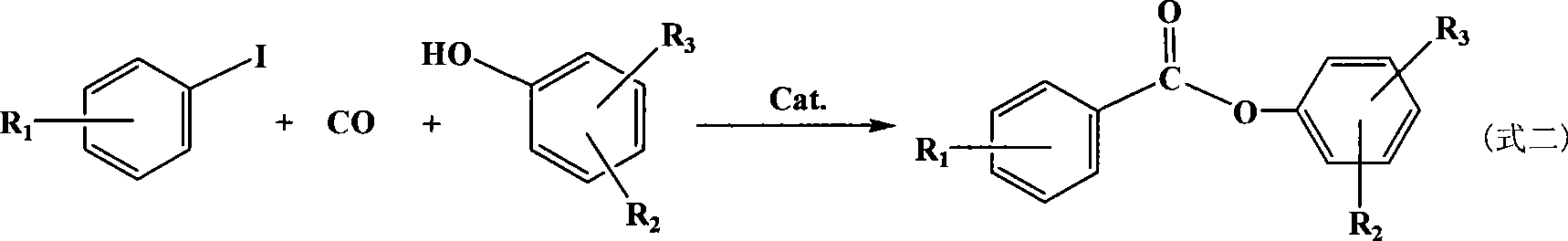
Method for synthesizing aromatic carboxylic ether by iodo aromatic hydrocarbon acarbonylation
A technology of aromatic carboxylic acid esters and iodoaromatics, which is applied in the field of halogenated benzene carbonylation to synthesize aromatic carboxylic acid ester compounds, can solve the problems of catalyst and product separation difficulties, loss of precious metals, pollution of phosphine compounds, etc., and achieve separation Easy, high reactivity, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 5%Pd / C: 5mg, Et 3 N: 7.2mmol, iodobenzene: 2.5mmol, anhydrous methanol 4mL, carbon monoxide pressure: 0.5MPa, react at 130°C for 2h to obtain methyl benzoate. The conversion rate of iodobenzene is 94%, and the selectivity of methyl benzoate is 100%.
Embodiment 2
[0030] With embodiment 1, used base is pyridine (7.2mmol), obtains methyl benzoate. The conversion rate of iodobenzene is 26%, and the selectivity of methyl benzoate is 100%.
Embodiment 3
[0032] With embodiment 1, do not add alkali, obtain methyl benzoate. The conversion rate of iodobenzene is 23%, and the selectivity of methyl benzoate is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com