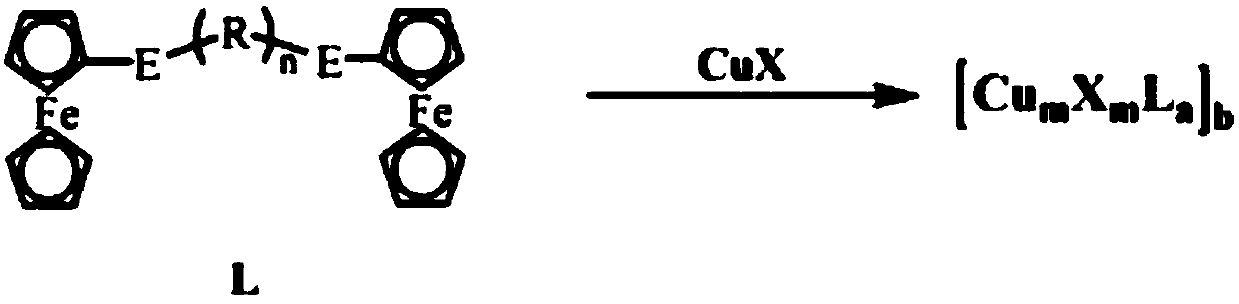Ferrocene cuprous cluster catalyst capable of catalyzing C-N coupled reaction and preparation method thereof
A coupling reaction, ferrocene technology, applied in the field of ferrocene cuprous cluster catalyst and its preparation, can solve the problems of harsh reaction conditions, low catalytic conversion rate, many extraction times, etc., and achieves rapid catalytic reaction and wide sources. , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Ferrocenyl Telluride Cuprous Cluster Thermal Catalyst 1 with Efficient Catalytic C-N Coupling (E=Te, n=3):
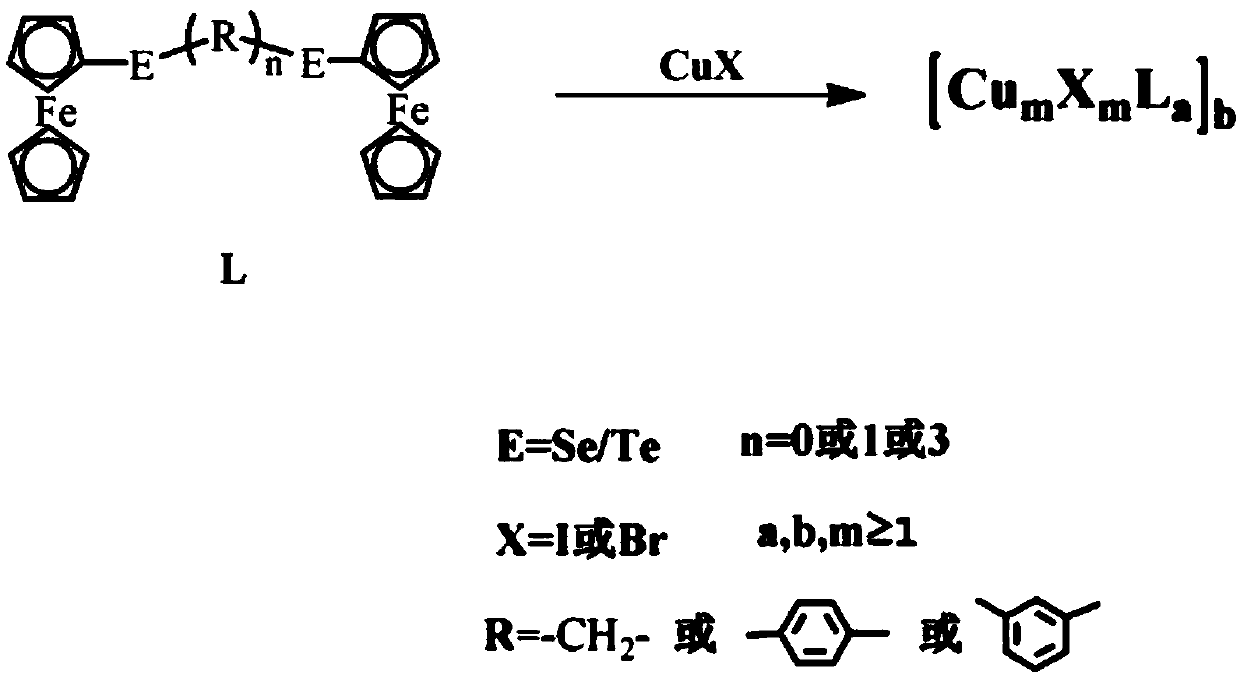
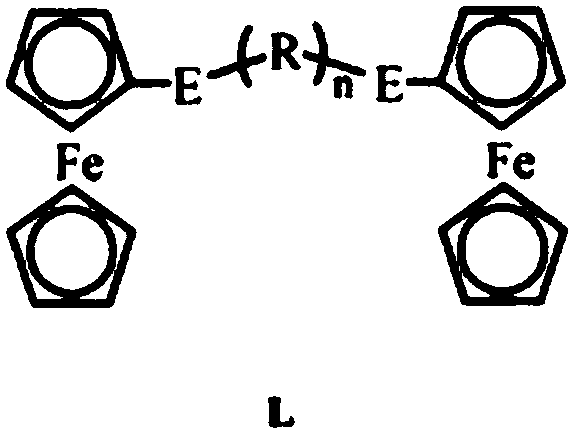
[0046] (1) Synthesis of ferrocenyl telluride ligand L (R=methylene, n=3, E=Te):
[0047] Under a nitrogen atmosphere, weigh 1 mmol of ferrocene and dissolve it in 200 ml of tetrahydrofuran, add 6 mmol of n-butyllithium and 1 mmol of tellurium powder in turn, react at room temperature, spin the reaction solution to dryness, and extract with dichloromethane. Purified by silica gel column chromatography, and collected Fc 2 Te 2 .
[0048] Take a 100mL three-neck flask, under nitrogen atmosphere, dissolve 0.5mmol ferrocene ditelluride into absolute ethanol, add 4mmol sodium borohydride and 0.5mmol 1,3-dibromopropane in turn, after the reaction is complete at room temperature, depressurize Distilled and spin-dried, and purified to obtain a yellow solid L.
[0049] (2) Synthesis of cuprous cluster 5 by interface method, (R=methylene, m=2, n=3, a=2, b...
Embodiment 2
[0053] Add 2.5mmol of iodobenzene, 0.375mmol of imidazole, 0.5mmol of lithium tert-butoxide, and 10% mol of cuprous iodide into the single-necked bottle, under nitrogen atmosphere, 110 degrees Celsius, and seal the reaction for 6 hours; the reaction stock solution is subjected to gas chromatography test, The product 1-phenylimidazole concentration was obtained. Yield was obtained after calculation.
Embodiment 3
[0055] In a 10ml single-necked bottle, add 2.5mmol of iodobenzene, 0.375mmol of imidazole, 0.5mmol of lithium tert-butoxide, catalyst 5 (E=Te, n=3, m=2, a=2, b=1, X=I) 10 %mol, under nitrogen atmosphere, 110 degrees centigrade, sealed reaction for 6 hours. After cooling down, the solids were separated and repeated experiments were performed. Repeat three times, carry out the gas phase test on the third reaction stock solution, and calculate the yield. The yields were 99.5%, 91.3%, 89.4%, respectively.
[0056] Table three embodiment 1,2,3 catalytic efficiency summary table
[0057] times
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com