Method of semi-synthesizing artemisinin
A kind of artemisinin and semi-synthetic technology, applied in the direction of organic chemistry, etc., can solve the problems of increasing the number of repeated operations, the limitation of the size of the reactor, and the expensive price of the reactor, etc., and achieve the effect of short process flow and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
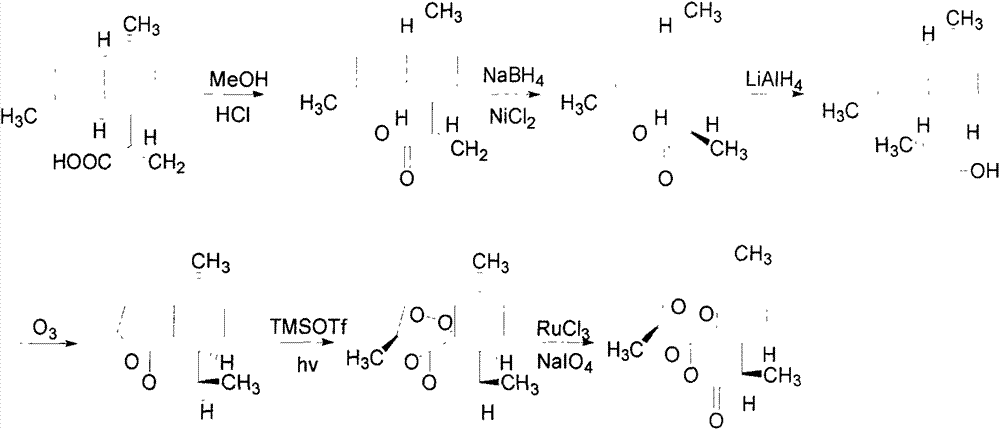
[0023] Synthesis of Dihydroartemisinic Acid
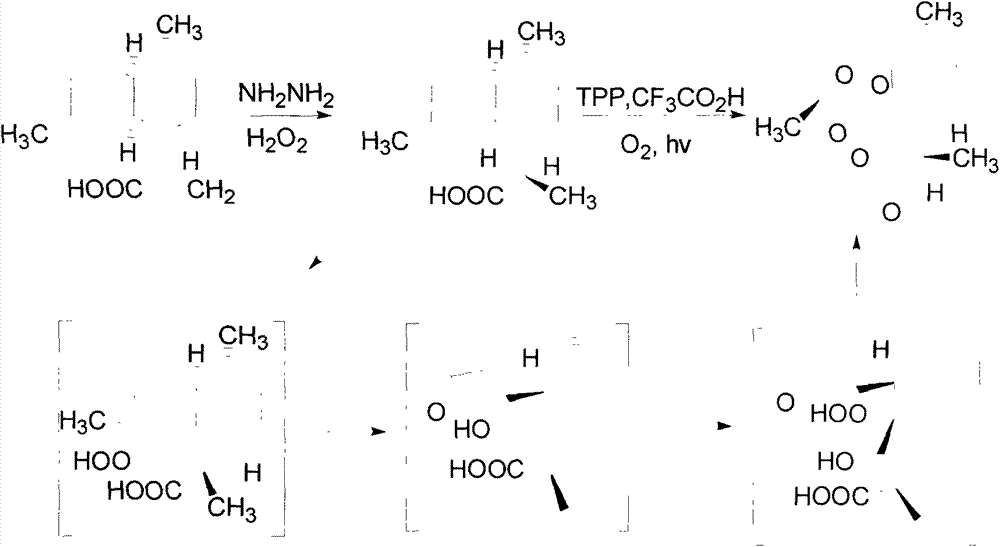
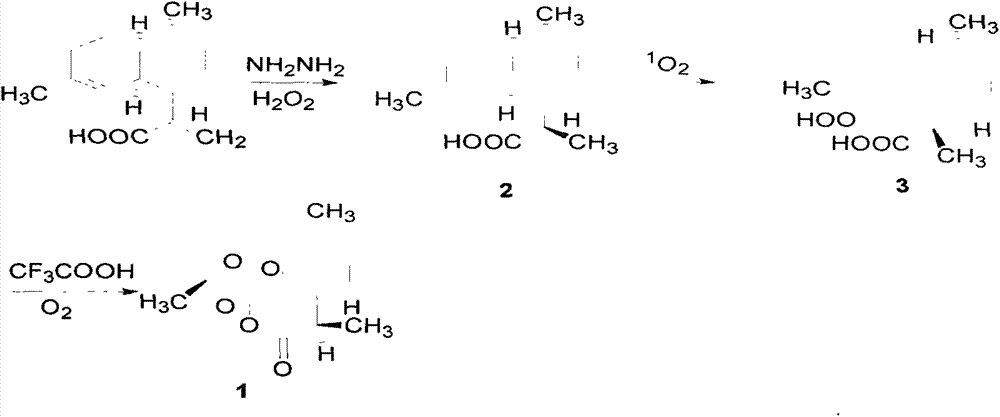
[0024] Dissolve 14.0 grams of artemisinic acid in 100 ml of absolute ethanol, add 10 ml of 80% hydrazine hydrate at room temperature, cool the system to 0 degrees with an ice bath, and then slowly add 50% hydrogen peroxide to the reaction system 10mL, the dropwise addition process was over 1 hour. The reaction was then allowed to stir overnight at room temperature. The reaction system was acidified with dilute hydrochloric acid until the pH value was about 2, extracted with methyl tert-butyl ether, dried over anhydrous magnesium sulfate, and concentrated to obtain 14.2 g of dihydroartemisinic acid with a yield of 100%.
[0025] Synthesis of Dihydroartemisinic Acid Peroxyalcohol
[0026] Add 14.2 grams of dihydroartemisinic acid, 300 milliliters of ethylene glycol and 100 milligrams of Na 2 MoO 4 . The reaction system was stirred at 60°C, and then 19.2 ml of 30% hydrogen peroxide solution was slowly added to the reaction system...
example 2
[0030] Synthesis of Dihydroartemisinic Acid
[0031] Dissolve 14.0 grams of artemisinic acid in 100 ml of absolute ethanol, add 10 ml of 80% hydrazine hydrate at room temperature, cool the system to 0 degrees with an ice bath, and then slowly add 50% hydrogen peroxide to the reaction system 10mL, the dropwise addition process was over 1 hour. The reaction was then allowed to stir overnight at room temperature. The reaction system was acidified with dilute hydrochloric acid until the pH value was about 2, extracted with methyl tert-butyl ether, dried over anhydrous magnesium sulfate, and concentrated to obtain 14.3 g of dihydroartemisinic acid with a yield of 100%.
[0032] Synthesis of Methyl Dihydroartemisinate
[0033] Add 14.3 g of dihydroartemisinic acid into a single-necked bottle, then add 100 ml of saturated methanolic hydrogen chloride solution, and reflux overnight. Then the reaction system was concentrated to obtain 15.0 g of methyl dihydroartemisinate.
[0034] ...
example 3
[0039] Synthesis of Dihydroartemisinic Acid
[0040] Dissolve 14.0 grams of artemisinic acid in 100 ml of absolute ethanol, add 10 ml of 80% hydrazine hydrate at room temperature, cool the system to 0 degrees with an ice bath, and then slowly add 50% hydrogen peroxide to the reaction system 10mL, the dropwise addition process was over 1 hour. The reaction was then allowed to stir overnight at room temperature. The reaction system was acidified with dilute hydrochloric acid until the pH value was about 2, extracted with methyl tert-butyl ether, dried over anhydrous magnesium sulfate, and concentrated to obtain 14.2 g of dihydroartemisinic acid with a yield of 100%.
[0041] Synthesis of Dihydroartemisinic Acid Peroxyalcohol
[0042] Into a three-necked flask, 14.2 grams of dihydroartemisinic acid, 300 milliliters of acetonitrile, 2 milliliters of acetone and 2 grams of potassium hydroxide were successively added. 19.2 ml of 30% hydrogen peroxide solution was slowly added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com