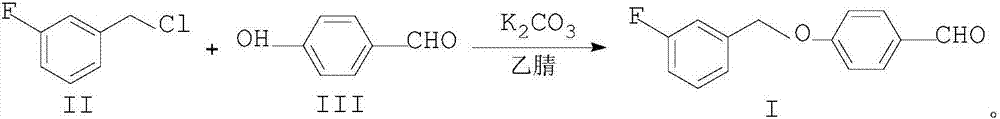
Preparation method of 4-(3-fluorine benzyloxy) benzaldehyde
A technology of fluorobenzyloxy and p-hydroxybenzaldehyde, which is applied in the field of preparation of 4-benzaldehyde, can solve problems such as complex processing methods, difficult crystallization, and difficulty in industrialization, and achieve stable process, simple post-processing method, and simplified operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 28.92g (200mmol) of compound II, 24.42g (200mmol) of compound III, 210mmol of potassium carbonate, and 150ml of acetonitrile, and heat up to reflux for 12h. The solvent was evaporated to obtain an oily product, which was added with 150 ml of water, stirred and cooled to crystallize, filtered at room temperature, and dried to obtain Compound I with a yield of 97.04% and an HPLC content of 99.53%.
[0027] The prepared compounds were tested, and the structural confirmation data are as follows:
[0028] MS(m / z): 231.0[M+H] + , 203.0[M-CO+H] +1 ;
[0029] HNMR: δ5.27(s, 2H), δ7.16(q, 3H), δ7.31(d, 2H), δ7.45(m, 1H), δ7.88(d, 2H), δ9.90 (s, 1H);
[0030] 13 CNMR: δ69.45(1C), δ114.94(1C), δ115.23(1C), δ115.38(2C), δ124.31(1C), δ130.63(1C), δ131.18(2C) , δ132.50 (1C), δ139.88 (1C), δ161.31 (1C), δ163.74 (1C), δ191.95 (1C).
Embodiment 2
[0032] Add 28.92g (200mmol) of compound II, 24.42g (200mmol) of compound III, 240mmol of sodium carbonate, and 290ml of acetonitrile, and heat up to reflux for 12 hours. The solvent was evaporated to obtain an oily product, which was added with 290 ml of water, stirred and cooled to crystallize, filtered at room temperature, and dried to obtain Compound I with a yield of 97.22% and an HPLC content of 99.47%.
Embodiment 3
[0034] Add 28.92g (200mmol) of compound II, 24.42g (200mmol) of compound III, 220mmol of sodium carbonate, and 250ml of acetonitrile, and heat up to reflux for 10h. The solvent was evaporated to obtain an oily substance, which was added with 250ml of water, stirred and cooled to crystallize, filtered at room temperature, and dried to obtain Compound I with a yield of 97.52% and an HPLC content of 99.55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com