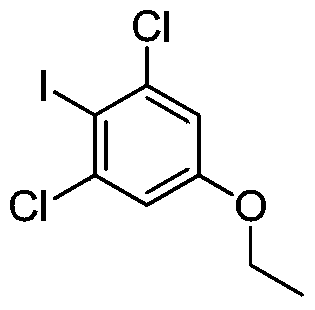
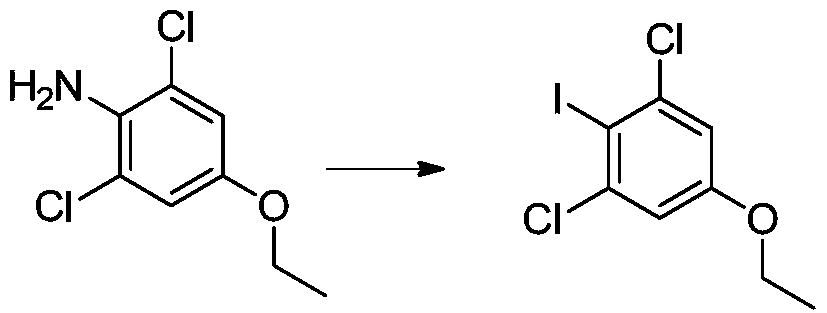
A kind of preparation method of 1,3-dichloro-5-ethoxy-2-iodobenzene
A technology of ethoxy and iodobenzene is applied in the field of preparation of compound intermediates, and can solve the problems of low crystallization temperature, high energy consumption, and difficulty in obtaining, and achieve the effects of high yield and high product content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
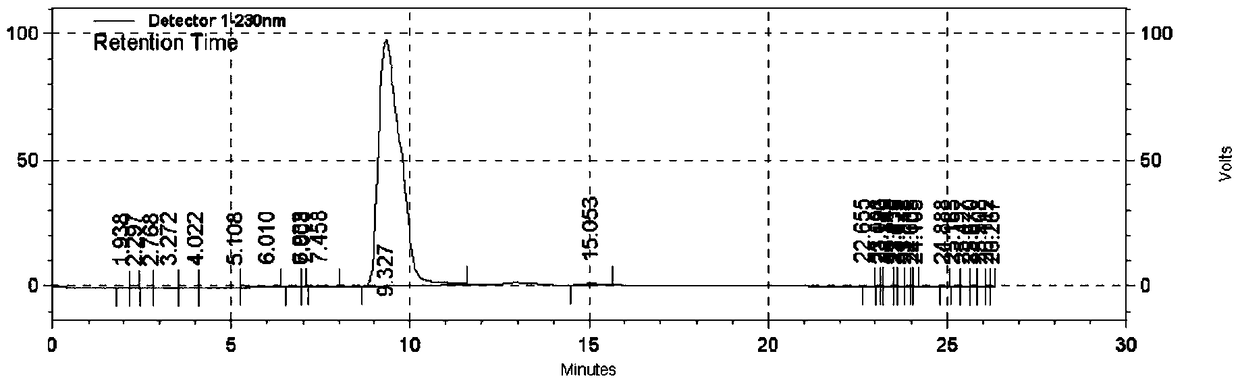
[0024] Add 40ml of concentrated sulfuric acid (mass percentage concentration 98%, the same below) into a 100ml four-necked bottle equipped with a rotor, and add 6.6g of sodium nitrite in batches under controlled internal temperature of 5-10 degrees. Stir for 10 min after addition.
[0025] Add 20g of 1,3-dichloro-5-ethoxy-2-aminobenzene and 100ml of formic acid in sequence to a 500ml four-necked bottle equipped with mechanical stirring, control the internal temperature at 8-12 degrees, and slowly add the prepared After the sulfuric acid solution of sodium nitrite was added dropwise, the temperature was slowly raised to 25 degrees at room temperature, and stirred for 2 hours. Add 1.2g of urea, this is the diazotization reaction solution.
[0026] Add 40g of potassium iodide and 40ml of water into another 500ml four-necked bottle equipped with mechanical stirring, and add the diazotization reaction solution dropwise at an internal temperature of 20-25 degrees. Sodium bisulfate...
Embodiment 2
[0028] Add 10ml of concentrated sulfuric acid into a 25ml single bottle equipped with a rotor, and add 2.0g of sodium nitrite in batches at an internal temperature of 5-10 degrees. Stir for 10 min after addition.
[0029] Add 5.0g of 1,3-dichloro-5-ethoxy-2-aminobenzene, 25ml of formic acid and 25ml of propionic acid in sequence in a 100ml four-necked bottle equipped with mechanical stirring, control the internal temperature at 8-12 degrees, and slowly Add the prepared sulfuric acid solution of sodium nitrite, after the dropwise addition, slowly warm up to room temperature 25°C, stir for 2 hours, and add 0.4g of urea. This is the diazotization reaction solution.
[0030] Add 10g of potassium iodide and 10ml of water into another 500ml four-necked bottle equipped with mechanical stirring, and add the diazotization reaction solution dropwise at an internal temperature of 20-25 degrees. Sodium bisulfate in 100ml of ice water, extracted with dichloromethane, separated, and conce...
Embodiment 3
[0032] Add 20ml of concentrated sulfuric acid into a 50ml four-necked bottle equipped with a rotor, and add 3.75g of sodium nitrite in batches at an internal temperature of 5-10 degrees. Stir for 10 min after addition.
[0033] Add 10g of 1,3-dichloro-5-ethoxy-2-aminobenzene and 100ml of acetic acid in sequence to a 250ml four-necked bottle equipped with mechanical stirring, control the internal temperature at 8-12 degrees, and slowly add the prepared After the sulfuric acid solution of sodium nitrite was added dropwise, the temperature was slowly raised to 25 degrees at room temperature, and stirred for 2 hours. Add 0.6g of urea, this is the diazotization reaction solution.
[0034] Add 20g of potassium iodide and 20ml of water into another 250ml four-necked bottle equipped with mechanical stirring, and add the diazotization reaction solution dropwise at an internal temperature of 20-25 degrees. Add 2.5g of 200-300 mesh silica gel to reflux for 30 minutes after adding 15ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com