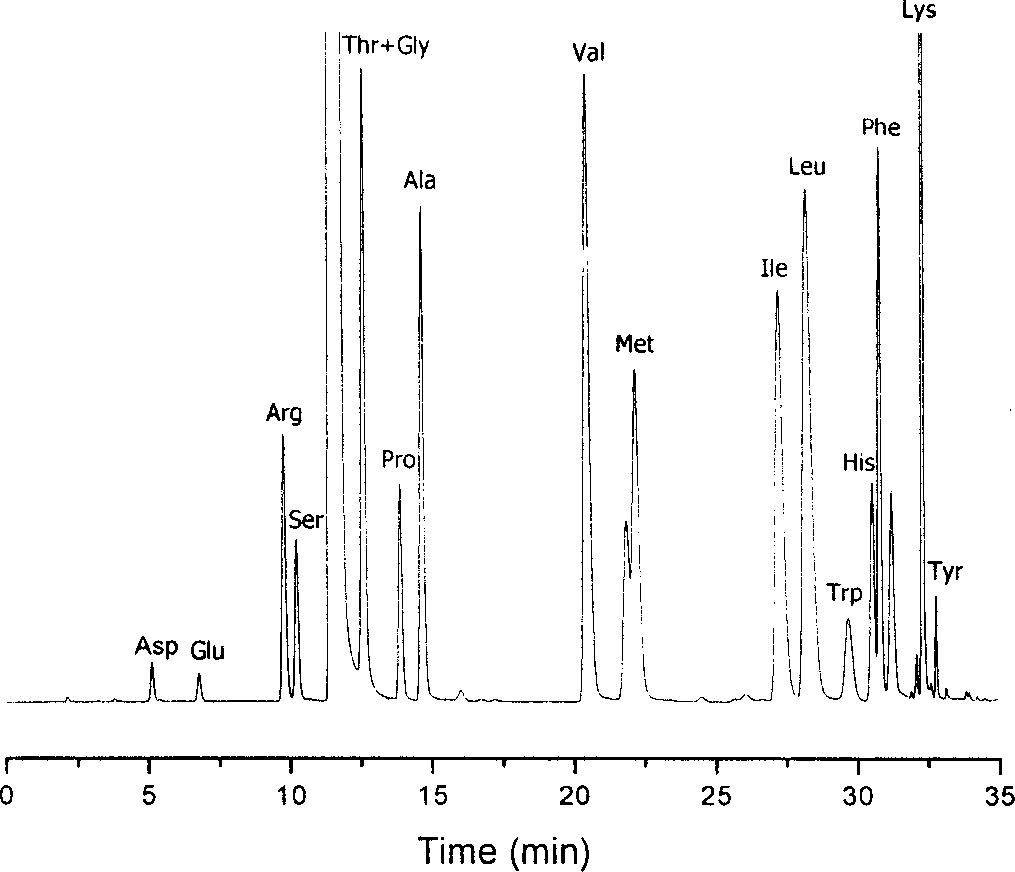
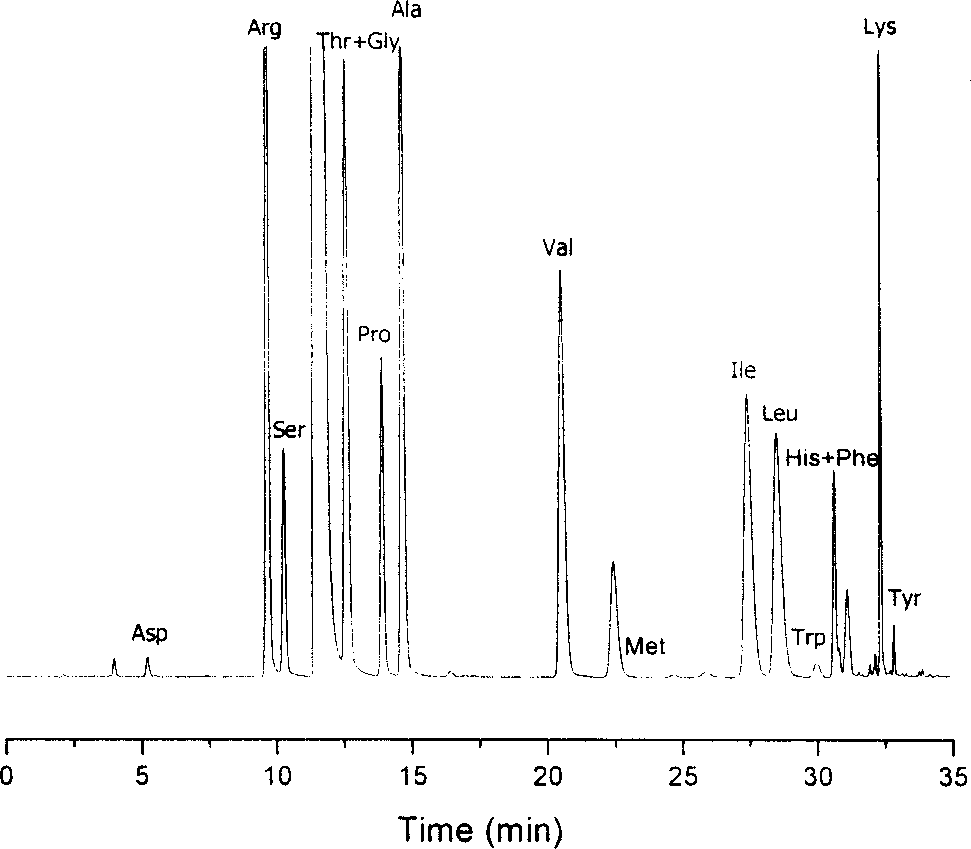
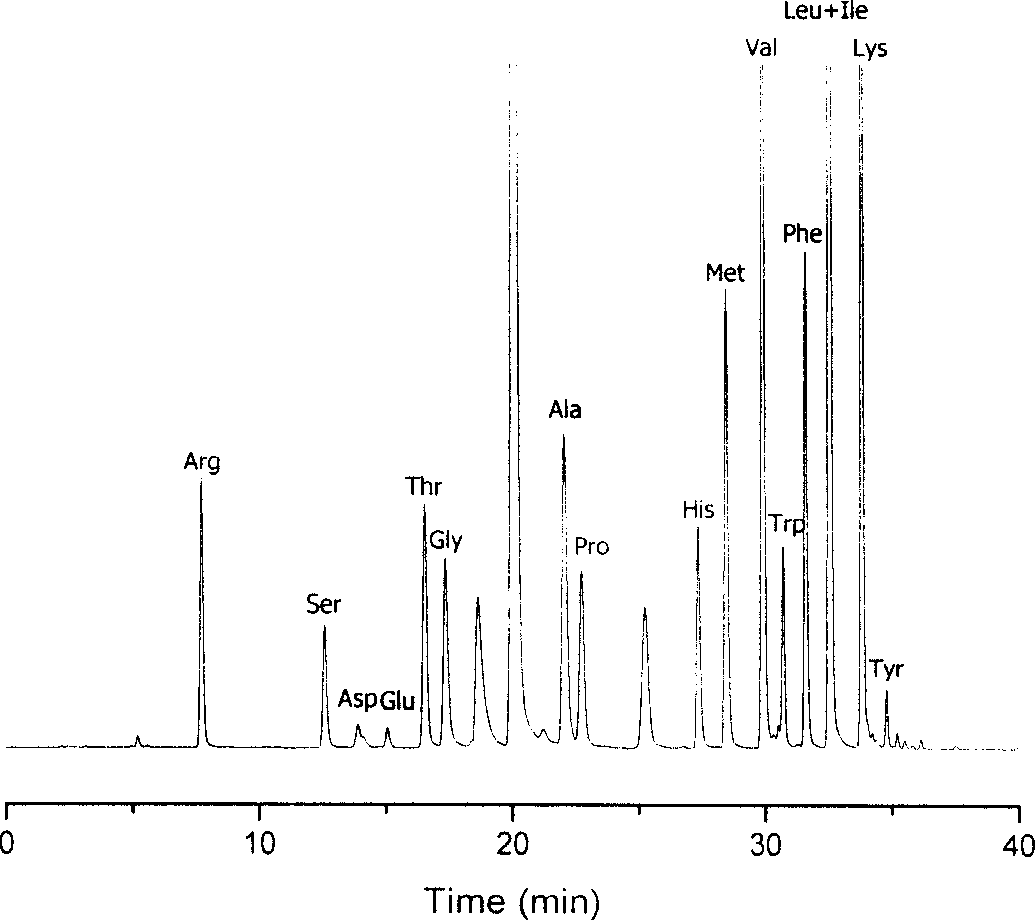
Method for analysizing amino acid
An analysis method and amino acid technology, applied in the field of amino acid analysis, can solve problems affecting the chromatographic separation results of DNFB-AA derivatives, etc., and achieve the effect of improving reproducibility and expanding the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 kidney disease injection (Amino acid for renal insufficiency, Hoechst Marion Produced by Roussel ) and liver disease injection (Amino acid preparation for hepatic insufficiency, Manufactured by Hoechst Marion Roussel Analysis of AA in )
[0048] 1. Reagents
[0049] 1.1.0.1M HCl 4mL hydrochloric acid mixed with 480mL water.
[0050] 1.2 1% DNFB 1g DNFB was dissolved in 100mL acetonitrile.
[0051] 1.3.0.5M NaHCO 3 2.1g NaHCO 3 Dissolve in 50mL water.
[0052] 1.4.0.1M phosphate buffer, pH6.5 Weighs 1.74g K 2 HPO 4 Dissolve in 100mL water to get 0.1MK 2 HPO 4 . Weigh 1.36g KH 2 PO 4 Dissolve in 100mL water to get 0.1M KH 2 PO 4 . To 0.1M KH 2 PO 4 Add 0.1M K 2 HPO 4 to pH=6.5.
[0053] 1.5.36mM triethylamine phosphate (TEAP), pH2.75 Take ~950mL water in a beaker, and add 5mL triethylamine to it with a pipette. After mixing, adjust the pH to 2.75 with phosphoric acid. Transfer the solution to a 1000mL volumetric flask and flush to t...
Embodiment 2
[0093] Example 2 Determination of content of main component propylglutamine in the bulk drug (produced by Tianjin Tiancheng Pharmaceutical Co., Ltd.) of propylglutamine (L-alanyl-L-glutamine)
[0094] 1. Reagents
[0095] 1.1. See Example 1.
[0096] 1. 2.6N HCl Concentrated hydrochloric acid and water are mixed in a ratio of 1:1 (V / V).
[0097] 2. Preparation of standard solution Take out the proglutamine reference substance from the desiccator, accurately weigh about 15mg into a polyethylene tube with a cover (1), add 1mL 6N HCl with a pipette, cover tightly, and shake well for later use.
[0098] 3. Preparation of sample solution Accurately weigh about 15 mg of proglutamine sample into a polyethylene tube with a cover (2), add 1 mL of 6N HCl with a pipette, cover tightly, and shake well for later use.
[0099] 4. Acid hydrolysis of standard solution and sample solution Heat polyethylene tubes (1) and (2) at 105°C for 6 hours. Let cool, shake well and set aside.
[0100]...
Embodiment 3
[0127] Determination of Proglutamine Content in Example 3 Proglutamine Injection (Sichuan Kelun Pharmaceutical Co., Ltd. Production)
[0128] 1. Reagents
[0129] 1.1. See Example 1.
[0130] 1.2.6.7N HCl 167mL concentrated hydrochloric acid diluted with water to 300mL.
[0131] 2. Preparation of standard solution Accurately weigh about 20mg of proglutamine or 13.5mg of glutamic acid reference substance into a polyethylene tube with a cover (1), add 100μL of water and 900μL of 6.7N HCl, cover tightly, and shake well for later use.
[0132] 3. Preparation of the sample solution Take 100 μL of the sample solution and 900 μL of 6.7N HCl in a polyethylene tube with a cover (2), cover it tightly, and shake well for later use.
[0133] 4. Acid hydrolysis Put the polyethylene tubes (1) and (2) in a heating block, heat at 105°C for 6 hours, take it out to cool, shake well and set aside.
[0134] 5. Derivatization reaction Use a pipette to draw 20mL each of the acid hydrolysis stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com