Enzymolysis-HPLC method for detecting enoxaparin
A technology of enoxaparin and enoxaparan enzyme, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve problems that are difficult to involve in the detailed structure of molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
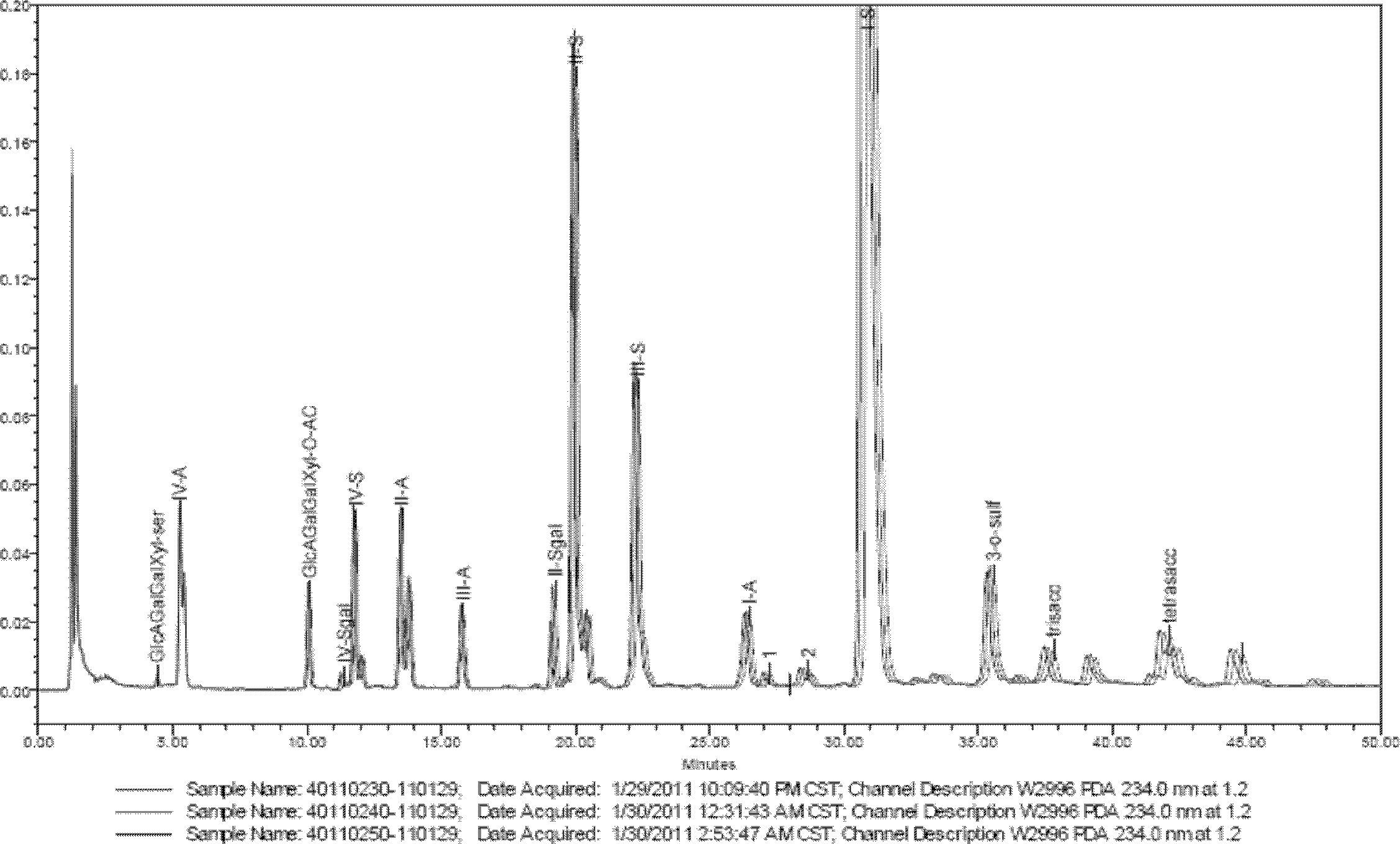
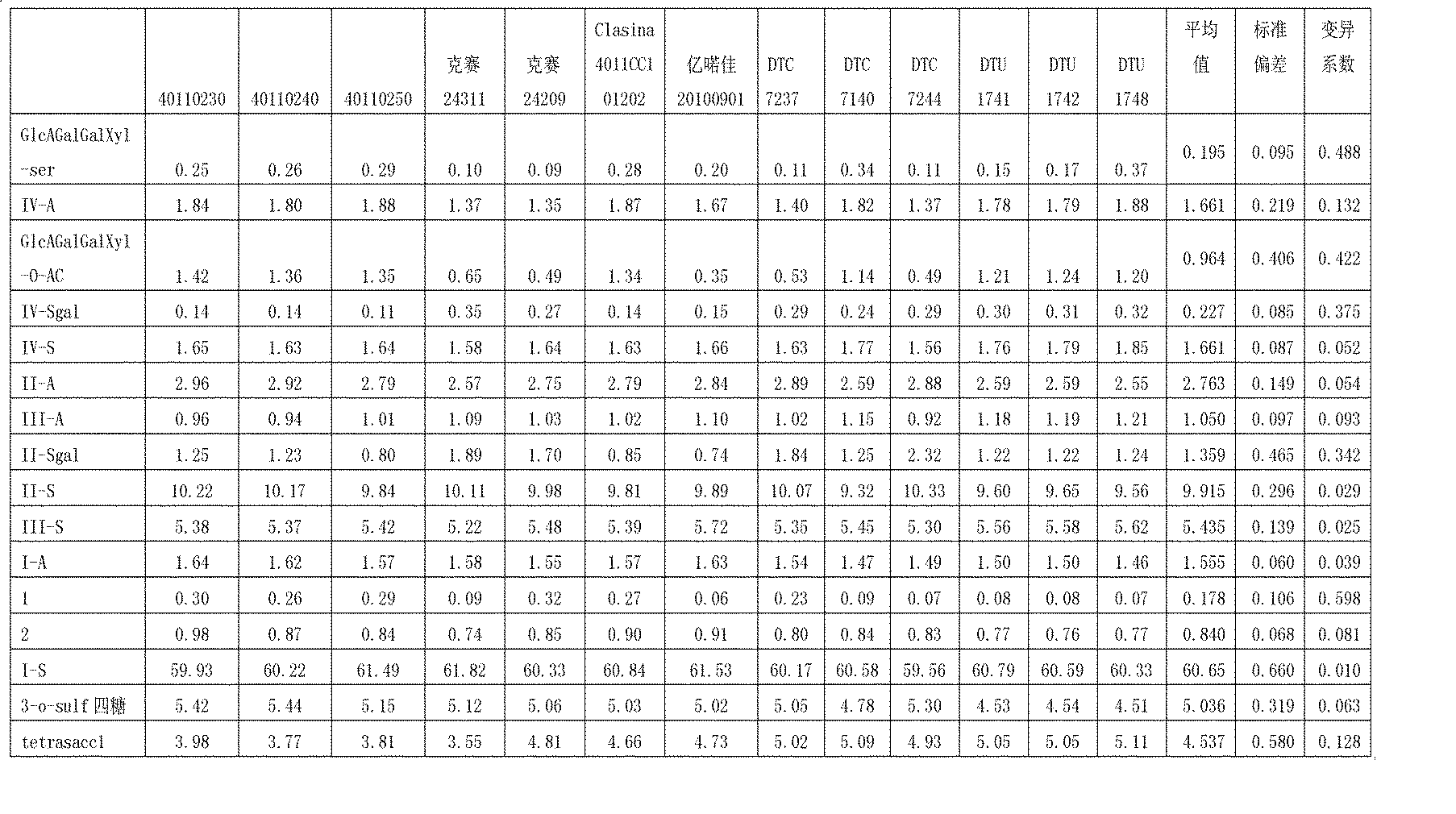
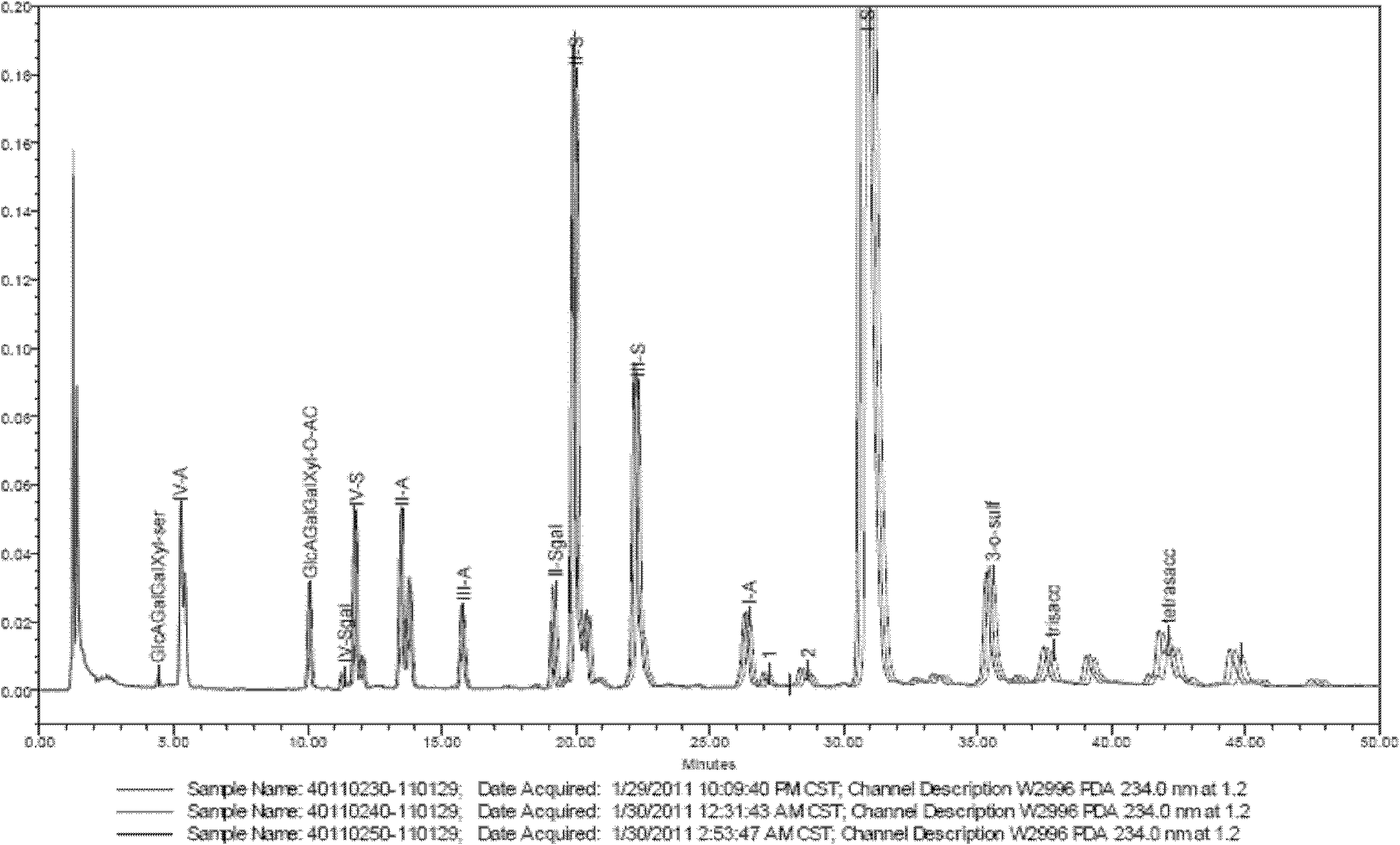
[0023] a. Solution preparation in the complete enzymatic hydrolysis step of enoxaparin:
[0024] Preparation of buffer solution A: Weigh 136mg of potassium dihydrogen phosphate, dissolve it with about 40ml of ultrapure water, add 50ml of glycerol, neutralize it with 1mol / Ld sodium hydroxide solution to pH 7, add 200mg The bovine albumin was transferred to a 100ml volumetric flask, made up to volume with ultrapure water, and shaken up. A 10 mM potassium dihydrogen phosphate solution was obtained.
[0025] Preparation of buffer B: pipette 0.57ml of glacial acetic acid into a beaker, add about 80ml of ultrapure water, add 35mg of calcium acetate, and dissolve it. Neutralize to pH 7 with 1 mol / L sodium hydroxide solution, and add 100 mg of bovine albumin. Transfer to a 100ml volumetric flask, dilute to the mark with ultrapure water, and shake well. A 100 mM sodium acetate solution was obtained.
[0026] Preparation of heparinase solution: Dilute heparinase to 1.5 IU / ml with bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com