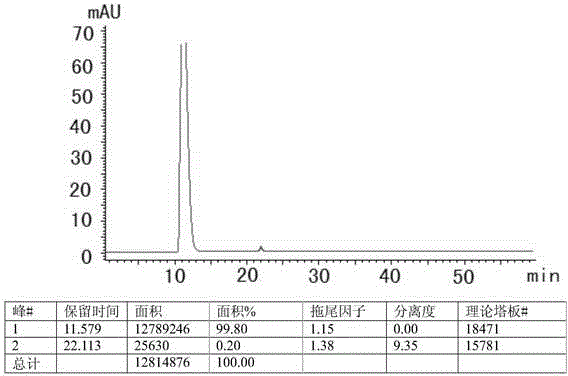
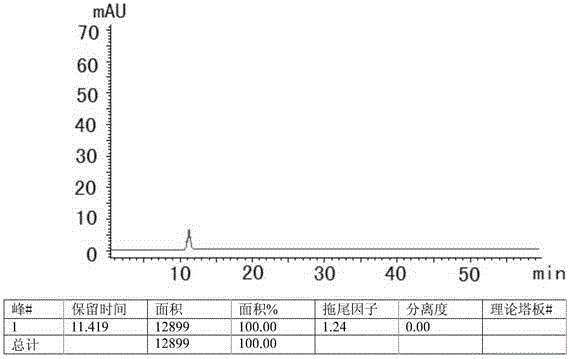
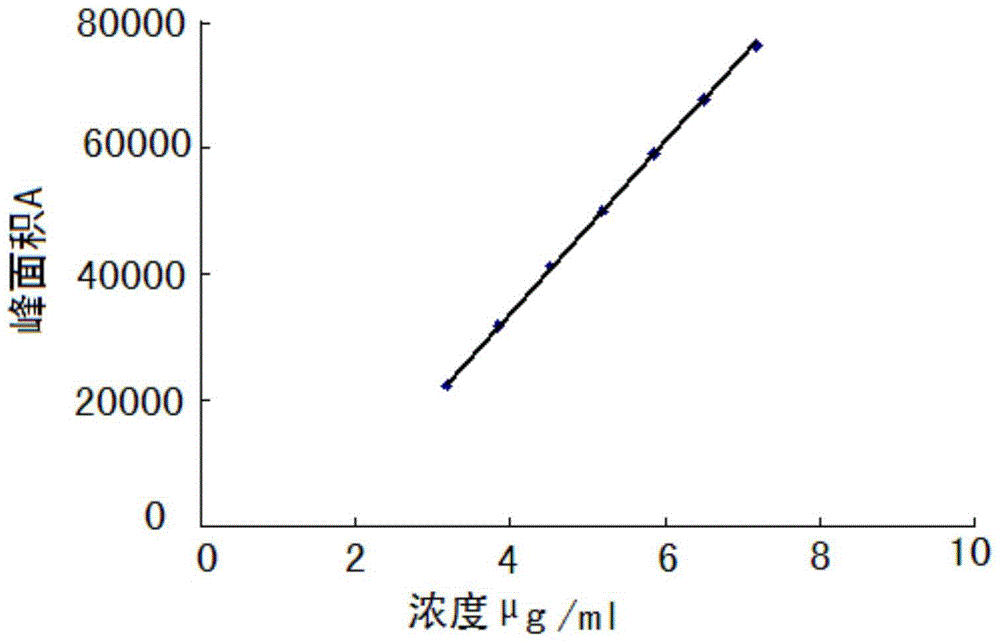
HPLC method for measuring related substances in Favipiravir
A technology for favipiravir and related substances, applied in the field of drug analysis and detection, can solve problems such as not yet retrieved, and achieve the effects of improving reliability, improving detection sensitivity, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1 Chromatographic conditions and system suitability test
[0023] 1.1 Selection of chromatographic conditions
[0024] Instrument: Shimadzu: LC-20AT, SPD-M20A, SIL-20A, DGU-20A5, the optimum column temperature is 30°C, and the flow rate is 1.0ml / min. The liquid chromatography column uses octadecylsilane bonded silica gel as the filler (250mm×4.6mm, 5μm). Referring to the relevant literature and combined with the specific conditions of the test, methanol-water, acetonitrile-water, methanol-acetonitrile-water, methanol-acetonitrile-water, and Various conditions such as methanol-acetonitrile-phosphate solution, and finally determine that the mobile phase is composed of acetonitrile (mobile phase A)-phosphate solution (mobile phase B), wherein the concentration range of phosphate solution (mobile phase B) is 0.005~0.015mmol / L, adjust the pH range from 6.0 to 8.0, the optimal concentration is 0.01mol / L, the optimal pH value is about 7.0, and carry out gradient elution acco...
Embodiment 2
[0039] Determination of related substances of embodiment 2 Favipiravir tablets
[0040] Take 20 tablets of this product, accurately weigh, grind finely, accurately weigh an appropriate amount (approximately equivalent to 2 mg of Favipiravir), add mobile phase to prepare a solution containing 0.2 mg per 1 ml, filter, and take the filtrate as the test sample Solution: Accurately measure an appropriate amount of the test solution, add mobile phase and dilute it to contain about 2 μg of solution per 1 ml, as a control solution. Under the following selected chromatographic conditions: diode array detector (Shimadzu: LC-20AT, SPD-M20A, SIL-20A, DGU-20A5), using octadecylsilane bonded silica gel as filler, mobile phase consists of Acetonitrile (mobile phase A)-phosphate solution (mobile phase B), wherein the concentration range of phosphate solution (mobile phase B) is 0.005-0.015mol / L, adjust the pH range to 6.0-8.0, and its optimal concentration is 0.01mol / L, the optimum pH value...
Embodiment 3
[0044] Determination of related substances of embodiment 3 Favipiravir capsules
[0045] Get an appropriate amount of the content of this product, grind it finely, precisely weigh the fine powder (approximately equivalent to 2 mg of Favipiravir), add mobile phase to prepare a solution containing 0.2 mg per 1 ml, filter, and take the filtrate as the test solution. Precisely measure an appropriate amount of the test solution, add mobile phase and dilute it to contain about 2 μg of solution per 1 ml, as a control solution. Under the following selected chromatographic conditions: a diode array detector (Shimadzu: LC-20AT, SPD-M20A, SIL-20A, DGU-20A5), using octadecylsilane bonded silica gel as a filler, and a mobile phase consisting of Acetonitrile (mobile phase A)-phosphate solution (mobile phase B), wherein the concentration range of phosphate solution (mobile phase B) is 0.005-0.015mol / L, adjust the pH range to 6.0-8.0, and its optimal concentration is 0.01mol / L, the optimum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com