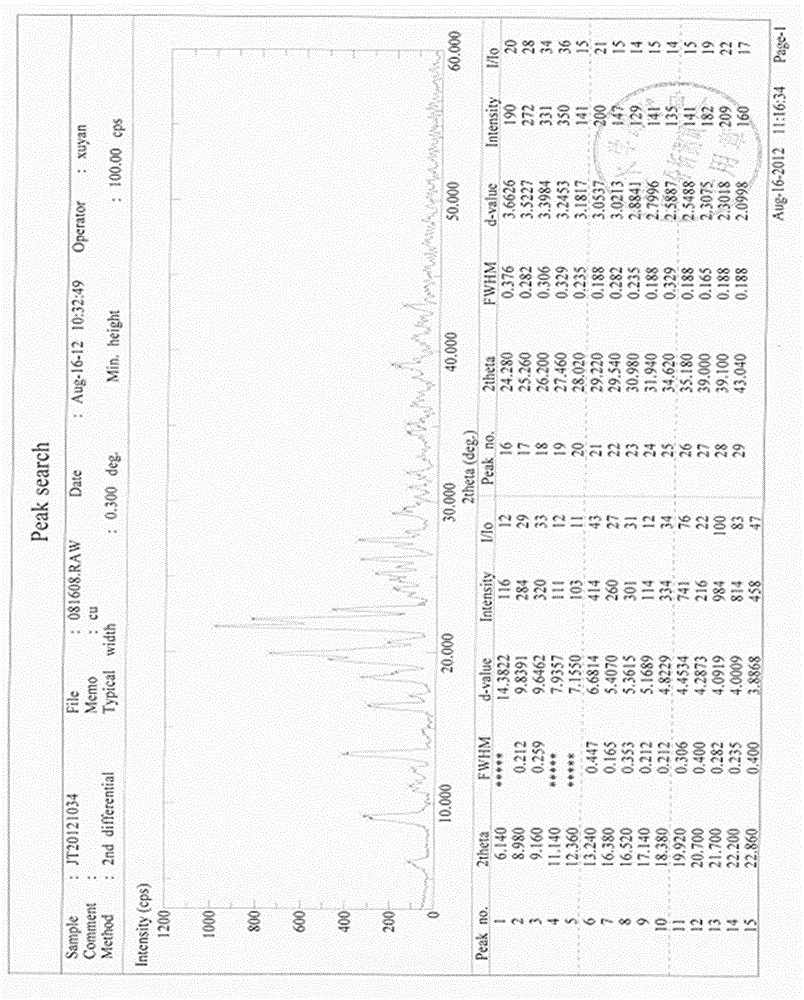
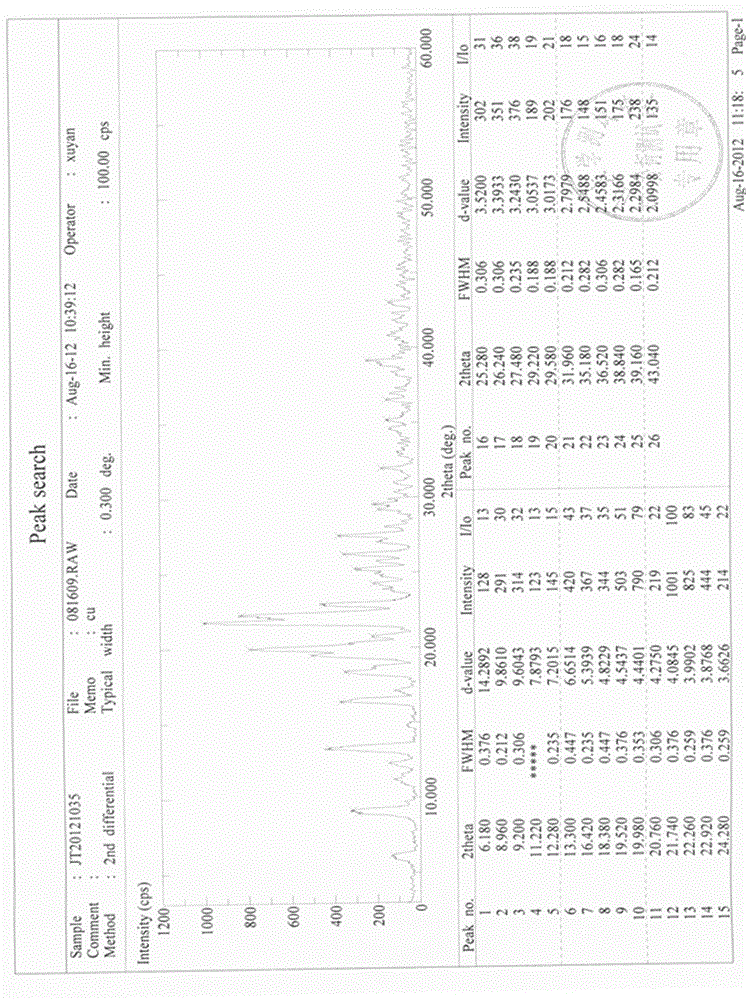
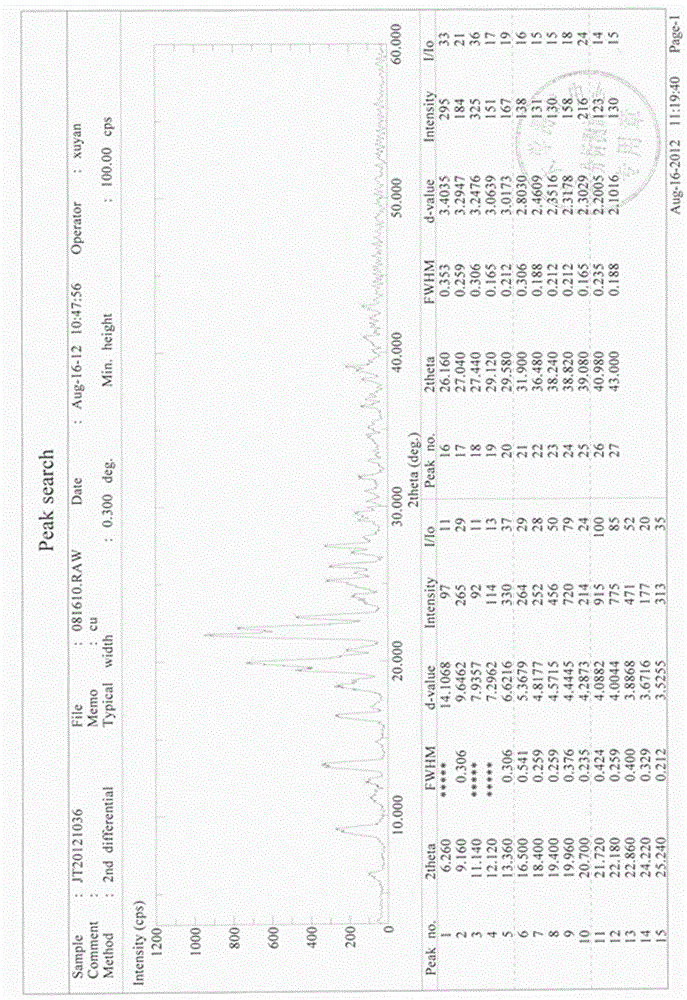
Stable gastrodin crystal with high bioavailability for oral administration as well as preparation method, preparation and application thereof
A gastrodin, utilization technology, applied in the field of medicine, to achieve the effect of high purity, good stability and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the stable gastrodin crystal with high oral bioavailability of the present invention comprises the following steps:
[0044] A, take gastrodin crude product, add the solvent that mass volume ratio is 1:1~200 to fully dissolve, filter and separate;
[0045] B. Add ethyl acetate with a volume ratio of 1:1 to 50 to the filtrate obtained in step A, stir, then stand for crystallization, and obtain the target product after centrifugation.
[0046] The solvent is one or more of water, 50-100% ethanol or 50-100% methanol.
[0047] The crystallization temperature is -10-40° C., and the crystallization time is 2-80 hours.
[0048] The preparation of the present invention is prepared into tablets or capsules by adding pharmaceutically acceptable carriers and auxiliary materials to the gastrodin crystals.
[0049] The application of the present invention is the application of the stable gastrodin crystal with high oral bioavailability in the preparation ...
Embodiment 1
[0072] Take 1kg of gastrodin to be refined, add 2L of water, heat to fully dissolve, and filter. Add 5L of ethyl acetate to the filtrate and stir well, then let it stand at 4°C for 20 hours to slowly precipitate crystals, filter, wash, and dry under reduced pressure at 60°C to obtain 0.78 kg of refined gastrodin. The recovery rate of the refining process is 78%, and the content is 99.07%.
Embodiment 2
[0074] Take 1kg of gastrodin to be refined, add 2L of 50% methanol aqueous solution, heat to fully dissolve, and filter. Add 5L of ethyl acetate to the filtrate and stir thoroughly, then stand at 25°C for 75 hours to slowly precipitate crystals, centrifuge the product, filter, wash, and dry under reduced pressure at 60°C to obtain 0.85 kg of refined gastrodin. The recovery rate of the refining process is 85%, and the content is 99.97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com