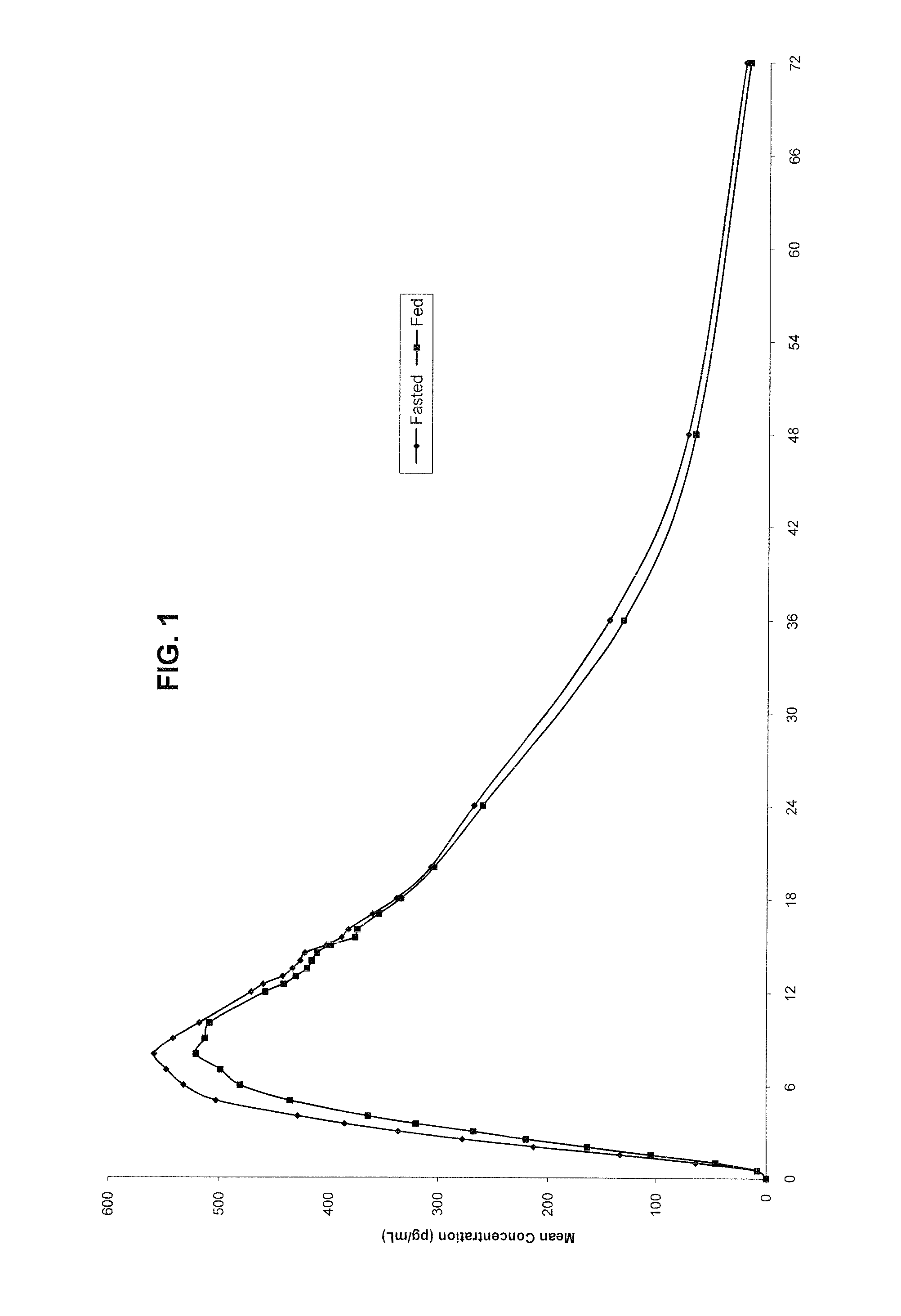
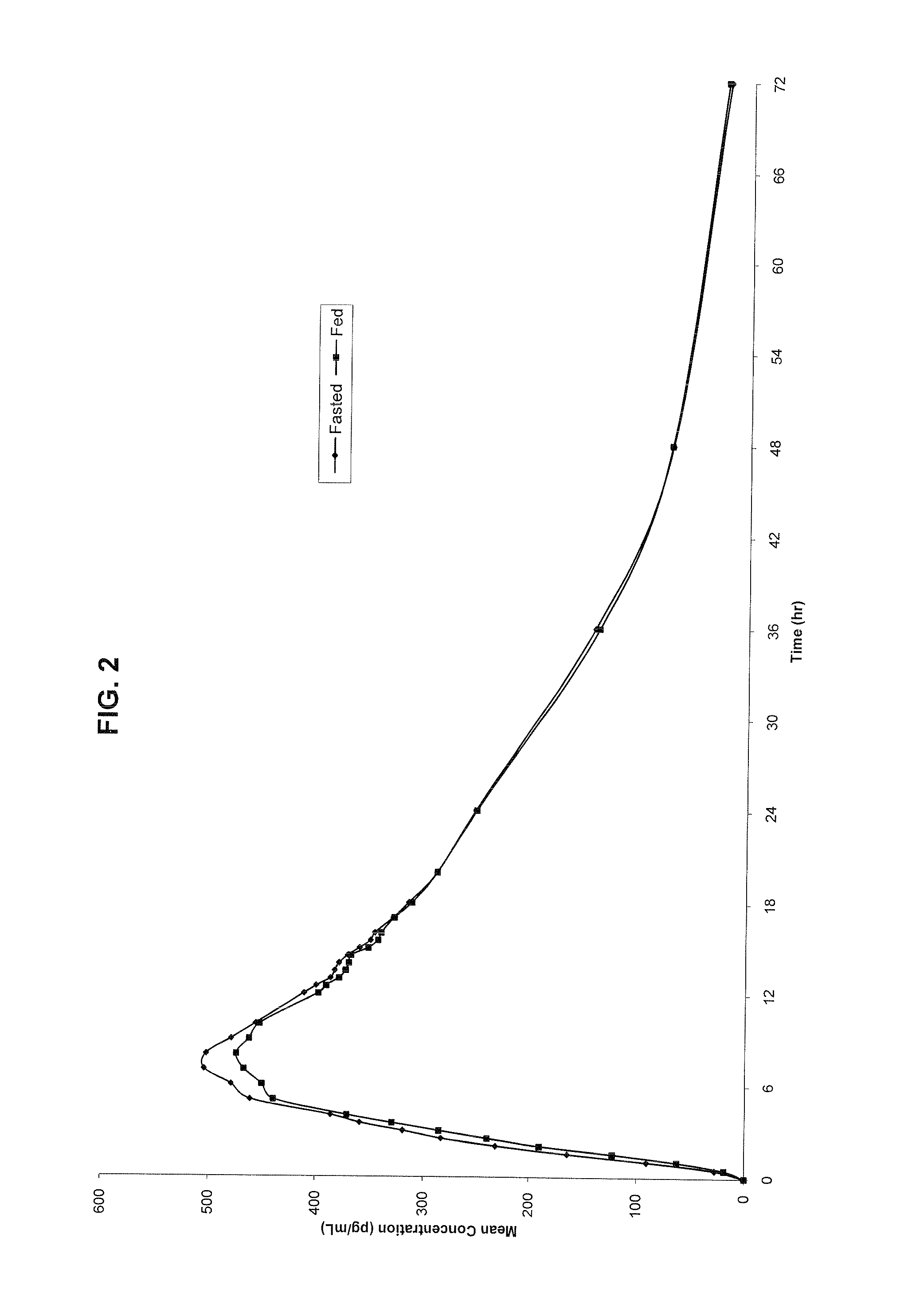
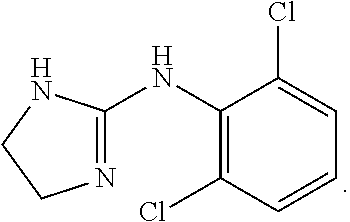
Novel clonidine formulation
a technology of clonidine and formulation, which is applied in the direction of biocide, animal repellents, dispersed delivery, etc., can solve the problems of unwanted sedating effects and failure to provide detailed illustrations of how, and achieve the effect of high tensile strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Clonidine Tablet Formulation: Barrier Coated Clonidine—Cation Exchange Resin Complex Matrix Modified Release Tablets, Equivalent to 0.2 mg Clonidine HCl
[0086]A. Preparation of Uncoated Clonidine—Cation Exchange Resin Complex Matrix
IngredientsGrams / batchClonidine HCl85Sodium Polystyrene Sulfonate12750(Amberlite ™ IRP-69) CationExchange ResinPovidone (Kollidon ® 30)1134Purified Water*qs**Removed during processing
[0087]The clonidine—resin complex matrix was prepared by first adding 80 L of purified water into a vessel and dissolving clonidine HCl therein by continuous mixing. A sodium polystyrene sulfonate ion exchange resin (AMBERLITE™ IRP-69) was dispersed with continuous mixing to form a slurry and the mixing was continued for 60 minutes to permit formation of a clonidine—ion exchange resin complex. Water from the slurry was removed by filtration. The wet resin complex was rinsed twice using purified water and then dried until the moisture content was about 10% to abo...
example 2
Barrier Coated Clonidine—Cation Exchange Resin Complex Matrix Tablets, Equivalent to 0.3 Mg Clonidine HCl
[0092]A. Preparation of Uncoated Clonidine—Cation Exchange Resin Complex Matrix
Ingredientsgms / BatchClonidine HCl85Sodium Polystyrene Sulfonate12750(Amberlite ™ IRP-69) CationExchange ResinPovidone (Kollidon ™ 30)1134Purified Water*Qs**Removed during processing
[0093]The clonidine—cation exchange resin complex was prepared by adding 80 L of purified water in to a vessel and then clonidine HCl, which was dissolved by continuous mixing. Sodium polystyrene sulfonate (AMBERLITE™ IRP-69) was dispersed in the vessel with continuous mixing to form a slurry and the mixing was continued for 60 minutes in order to permit formation of the clonidine—cation exchange resin complex. Water from the slurry was removed by filtration process. The wet clonidine—ion exchange resin complex was rinsed twice using purified water. This wet resin complex was then dried until the moisture content was about 1...
example 3
Preparation of Barrier Coated Clonidine—Ion Exchange Resin Complex Matrix Liquid Suspension Formulation
[0098]A. Preparation of Uncoated Clonidine—Cation Exchange Resin Complex Matrix
Ingredientsgms / BatchClonidine HCl85Sodium Polystyrene Sulfonate12750(Amberlite ™ IRP-69) CationExchange ResinPovidone (Kollidon ™ 30)1134Purified Water*Qs**Removed during processing
[0099]The clonidine—cation exchange resin complex was prepared by first adding into a vessel 80 L of Purified Water and dissolving clonidine HCl therein by continuous mixing. A sodium polystyrene sulfonate cation exchange resin (AMBERLITE™ IRP-69) was dispersed with continuous mixing to form a slurry and the mixing was continued for 60 minutes in order to permit complexation of the clonidine and the cation exchange resin. Water from slurry was removed by filtration process. The wet clonidine—cation exchange resin complex was rinsed twice using purified water. This wet resin complex was then dried until the moisture content was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation | aaaaa | aaaaa |
| elongation factor | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com