Method of treating psoriasis using anti-gamma interferon antibody
a technology of gamma interferon and anti-psoriasis, which is applied in the field of treating psoriasis using gamma interferon antibody, can solve the problems that the model lacks certain histological hallmarks of the human body, and achieves the effect of improving the histological quality of the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0067] Materials and Methods
[0068] Mice. Female BALB / cj mice and BALB / cj-IFN.gamma..sup.-1- mice (donor mice) were purchased from Jackson Labs (Bar Harbor, Me.). C.B-17 / 1cr scid / scid mice and C.B-17 scid-beige double mutant mice (recipient mice) were purchased from Taconic (Germantown, N.Y.). All mice were housed in a specific pathogen free environment at the Protein Design Labs animal facility and were used between 4-12 weeks of age. Sentinel mice were used to screen for the following pathogens: MHV, sendai, PVM, REO3, TMEV GDVIII, M. pulmonis and parvovirus. Random screens of mice for pinworms were also conducted. None of the pathogens listed above were detected at anytime. Mice were housed 2-5 per microsisolator. All scid / scid or scid / beige mice were handled with gloves under a class II hood, fed sterile food and water ad libitum, and maintained inside a laminar flow tent (Bioclean Maywood, N.J.) in sterile microisolators that were changed weekly. Donor mice were housed in conven...
example 2
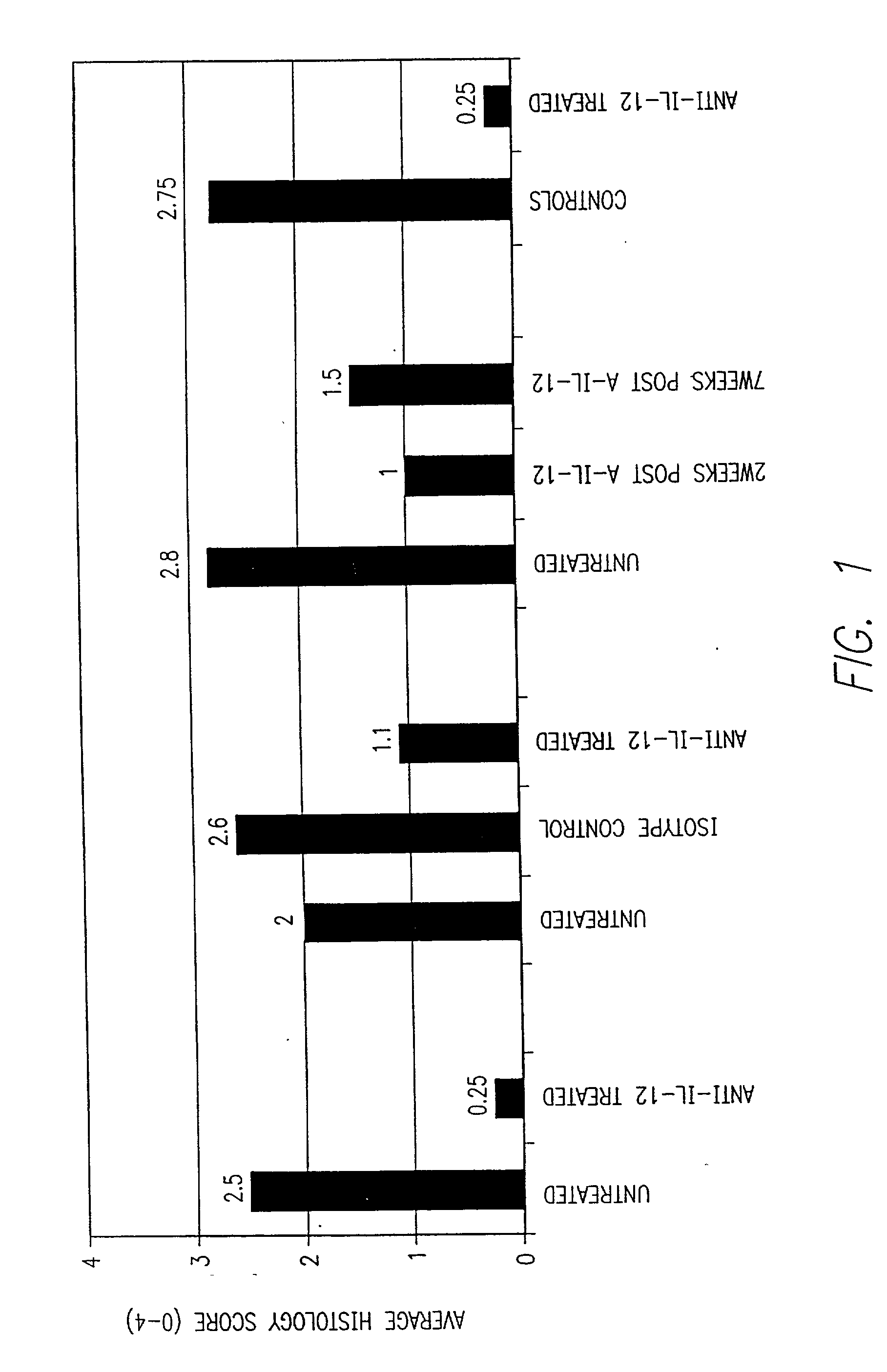
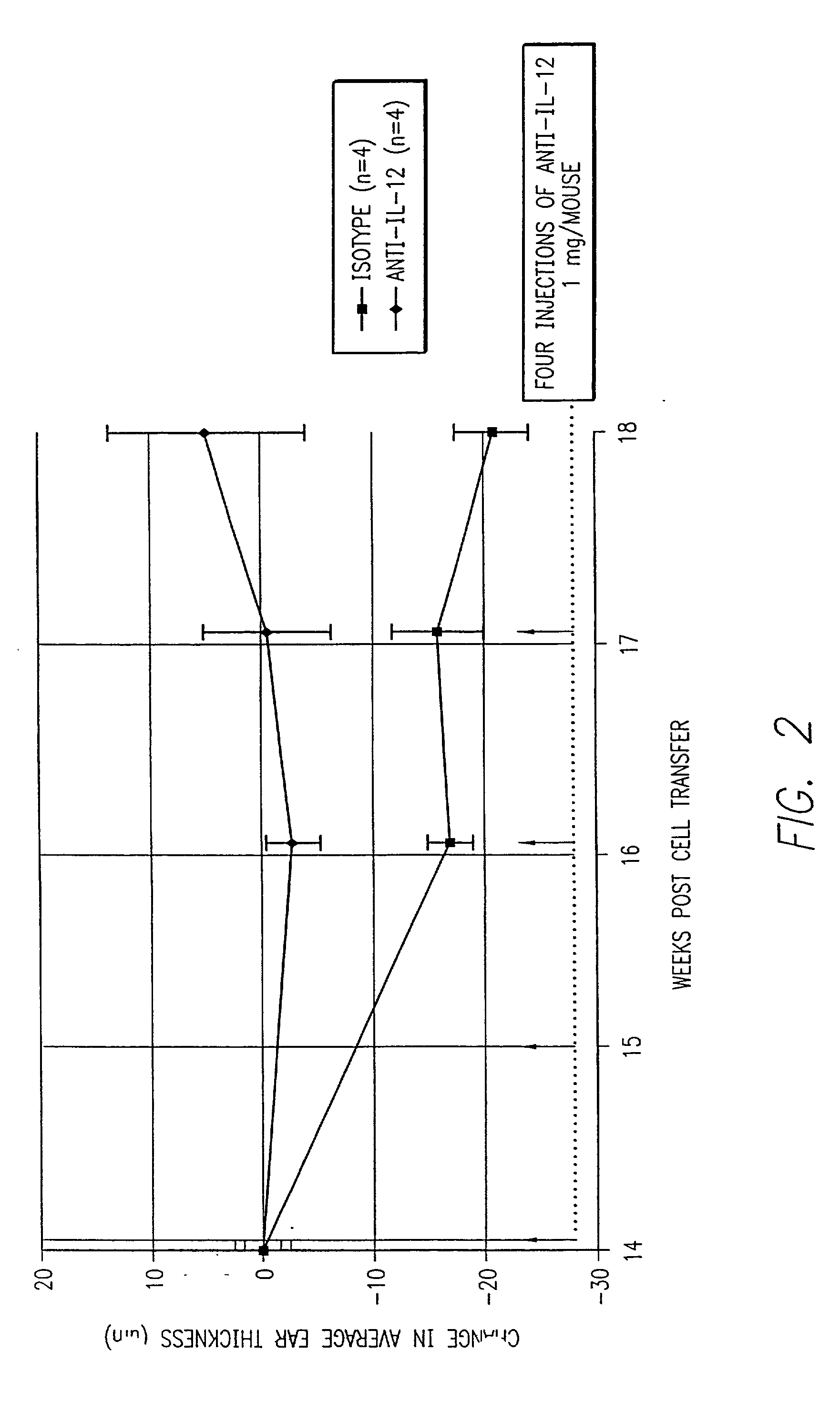
[0102] Animals were tested for the effect of treatment with antibodies to IL-12 on on going and late-stage psoriatic disease. The disease was induced by the methods described in Example 1, i.e., using 3.times.10.sup.5 CD4.sup.+CD45Rb.sup.hi cells transferred into scid / scid mice followed by injection of LPS and IL-12. Treatment with the anti-IL-12 antibody was always at 1 mg / mouse / dose. The results of four separate experiments are shown in FIG. 1. In the initial experiment, (n=5 untreated; n=2 treated), the mice received injections of anti-IL-12 at weeks 9 and 10 (i.e., about 5 weeks after the appearance of psoriatic symptoms) and were sacrificed for histology at week 14. Whereas the untreated mice had an average histology score of 2.5, indicating moderate to high symptoms, the treated mice had a histology score of only 0.25, indicating almost no symptoms. Hence, two injections of the anti-IL-12 antibody were highly effective in alleviating established symptoms of psoriasis.
[0103] In...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com