Anti-human interleukin 5 (1L-5) monoclonal antibody and application thereof
An antibody, recombinant protein technology, applied in the field of biomedicine, can solve problems such as lack of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0306] The preparation method of the nucleic acid is a conventional preparation method in the art. Preferably, it includes the following steps: obtaining the nucleic acid molecule encoding the above-mentioned protein by gene cloning technology, or obtaining the nucleic acid molecule encoding the above-mentioned protein by artificial full-sequence synthesis .
[0307] Those skilled in the art know that substitutions, deletions, alterations, insertions or additions can be appropriately introduced into the base sequence encoding the amino acid sequence of the above protein to provide a polynucleotide homologue. The homologue of the polynucleotide in the present invention can be prepared by replacing, deleting or adding one or more bases in the gene encoding the protein sequence within the scope of maintaining antibody activity.
[0308] carrier
[0309] The invention also provides a recombinant expression vector comprising the nucleic acid.
[0310] The recombinant expression v...
Embodiment 1
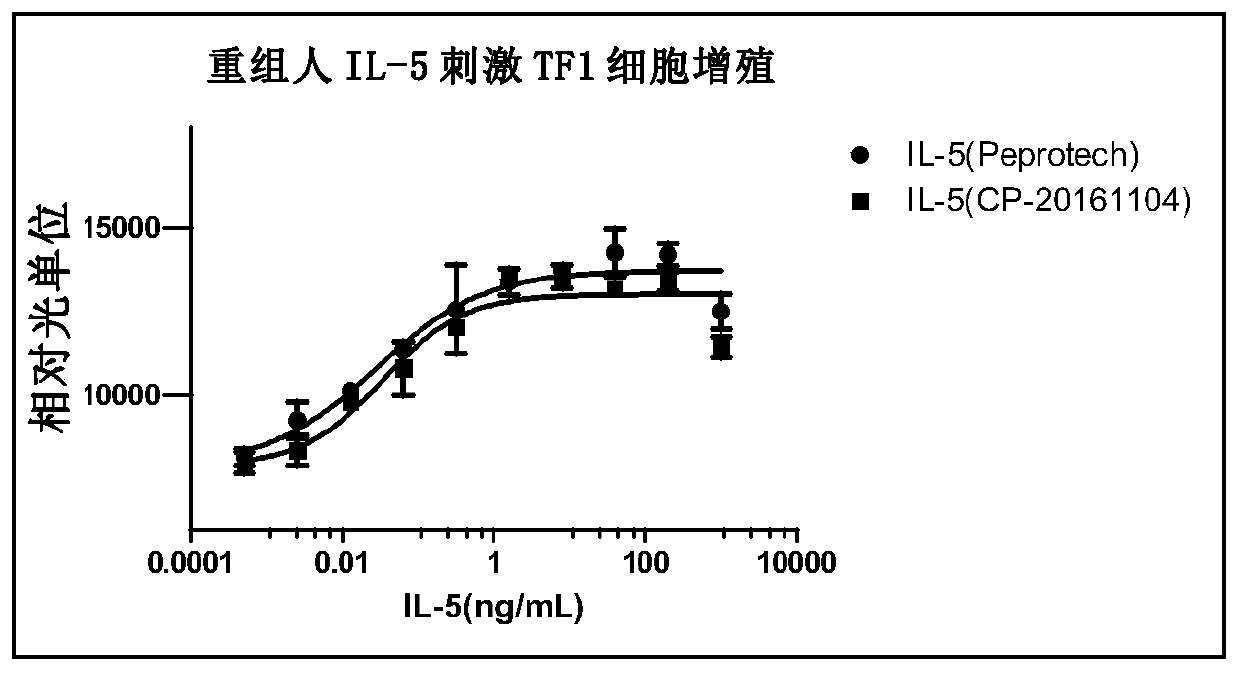
[0383] Example 1: Expression and purification of recombinant human IL-5
[0384] Using the PCR method, the 3' end of the DNA fragment encoding human IL-5 protein amino acid sequence (NP_000870.1) Met1-Ser134 was added to the DNA encoding six histidines, and the obtained encoding his tag recombinant human IL-5 protein The DNA fragments were cloned into expression vectors by molecular biology methods. Escherichia coli is used to amplify, the plasmid is extracted and purified by alkaline lysis, and the expression plasmid is transiently transfected to express the recombinant protein using insect cell SF21. After 5-7 days of culture, centrifuge and filter to collect the cell culture supernatant. The recombinant human IL-5 protein with His tag in the supernatant was purified by Ni-NTA affinity chromatography, and then further purified by using a molecular sieve column to remove impurities such as macromolecular polymers. The purified protein was stored in PBS buffer, filtered thro...
Embodiment 2
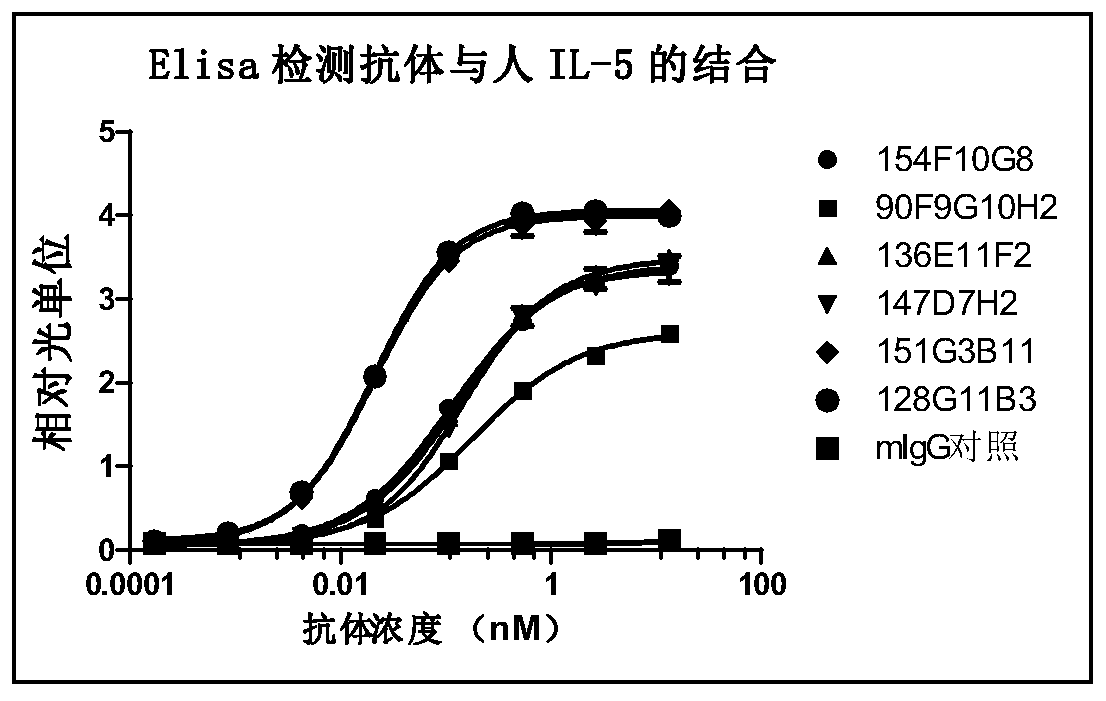
[0389] Example 2: Preparation of anti-human IL-5 antibody using hybridoma technology
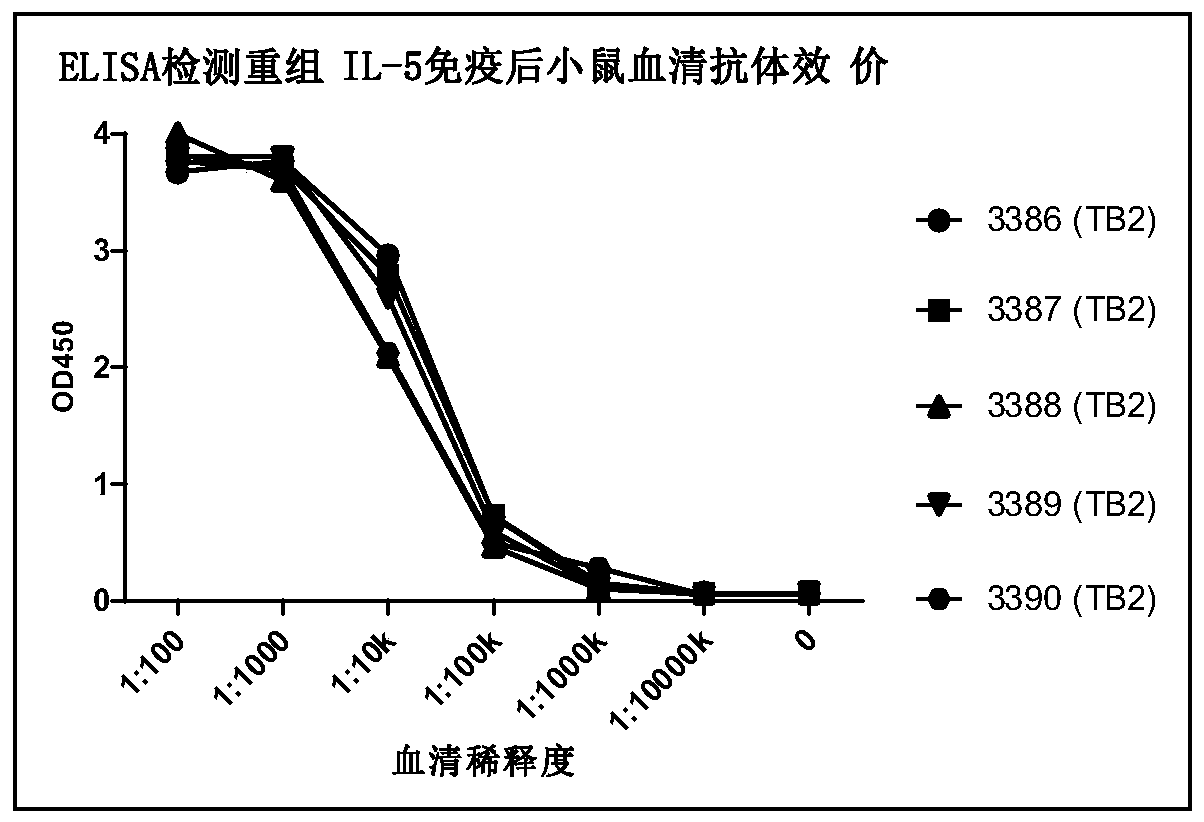
[0390] 2.1 Mice were immunized with recombinant human IL-5 protein
[0391] For protein immunization, 6-8 week-old Balb / c, SJL / J mice (provided by Shanghai SLAC) were used. Mice were reared under SPF conditions after receipt. The initial immunization dose was 50 micrograms of recombinant human IL-5 protein per mouse. After the protein was emulsified with complete Freund's adjuvant, 0.25 ml was injected subcutaneously into the tail. Two weeks after the initial immunization, a booster immunization was given. Recombinant human IL-5 protein (25 μg protein per mouse) was emulsified with Freund's incomplete adjuvant and injected 0.25 ml intraperitoneally. After each booster immunization interval of 3 weeks. Serum samples were collected one week after each booster immunization, the antibody titer in the serum was detected by ELISA and the activity of the antibody in the serum was detected by r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com