Method for preparing recombinant human granulocyte colony stimulating factors screened by microorganisms, and medicament composition and preparation thereof
A colony-stimulating factor and granulocyte technology, which is applied in the direction of colony-stimulating factor, drug combination, peptide preparation method, etc., can solve the problem that the mass recovery rate is less than 20%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
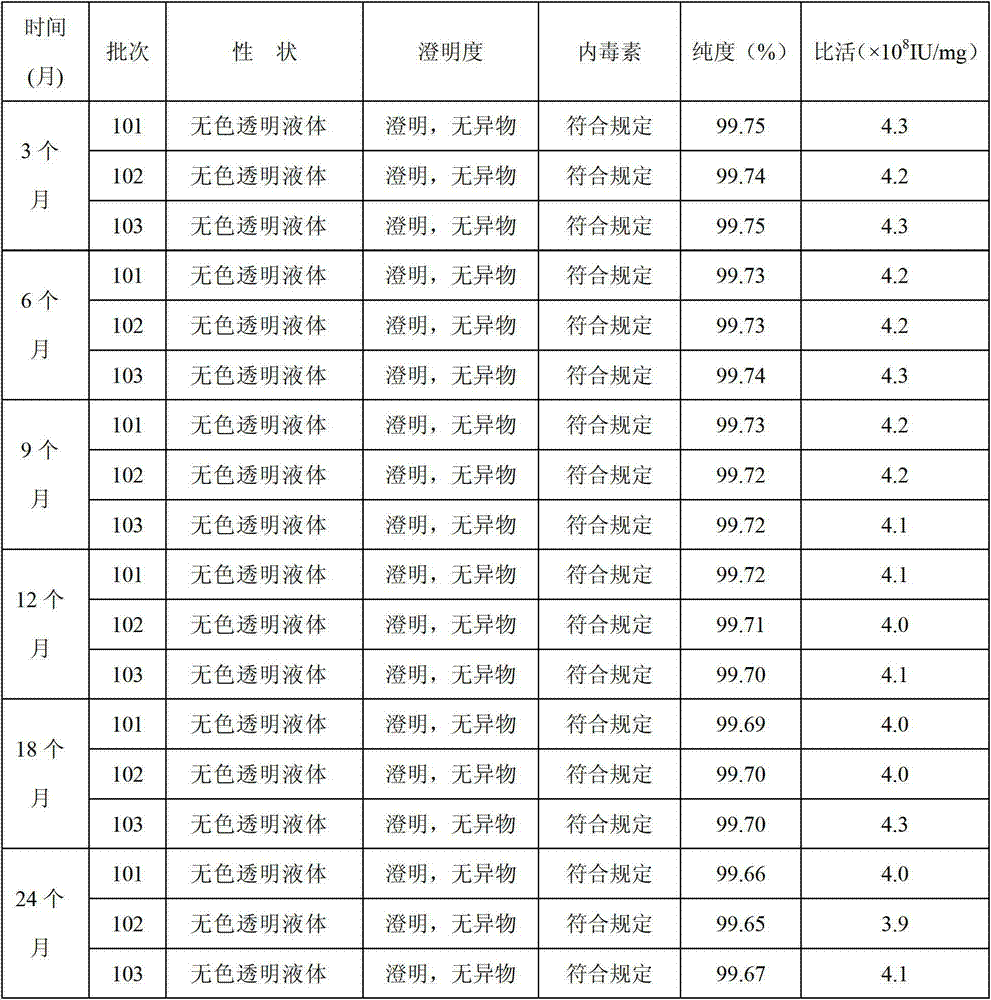
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of recombinant human granulocyte colony-stimulating factor:
[0032] 1. Select engineering bacteria DH5α-PBV220-hGCSF strain for fermentation;
[0033] 2. Wash the collected cells with TE buffer for 3 times, then ultrasonically crush for 0.5-1 hour, centrifuge at low temperature, and collect inclusion bodies; then add inclusion body washing solution, mix at 4°C for 30 minutes, and centrifuge at 4°C at low temperature ; TE buffer: 20mmol / L Tris-HCl+1mmol / L EDTA, pH8.0; inclusion body washing solution: 20mmol / L Tris-HCl+1mmol / L EDTA+2mmol / L urea+0.5%Tritonx-100, pH8.0; the volume ratio of inclusion body weight to inclusion body washing solution is 1:10;
[0034] 3. Add inclusion body solution to the inclusion body, centrifuge at 4°C for 12 hours to obtain inclusion body extract; the inclusion body solution is: 20mmol / L Tris-HCl+1mmol / L EDTA+7mmol / L Guanidine hydrochloride + 20mmol / L DTT, pH8.0; the ratio of inclusion body mass to inclusion body sol...
experiment example 1
[0043] Experimental example 1: The effect of urea concentration in the eluent of SephacrylS-200 gel column on hG-GSF
[0044] Change the concentration of urea in the eluate in Example 1 (other conditions and steps are the same as in Example 1), and study its influence on the yield and specific activity of recombinant human cell colony-stimulating factor, and the results are shown in Table 1:
[0045] Table 1:
[0046]
[0047] Urea (mmol / L)
experiment example 2
[0048] Experimental example 2: Effect of pH of eluent in SephacrylS-200 gel column on hG-GSF
[0049] Change the pH in the eluate in Example 1 (other conditions and steps are the same as in Example 1), and study its influence on the yield and specific activity of recombinant human cell colony-stimulating factor, and the results are shown in Table 2:
[0050] Table 2:
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com