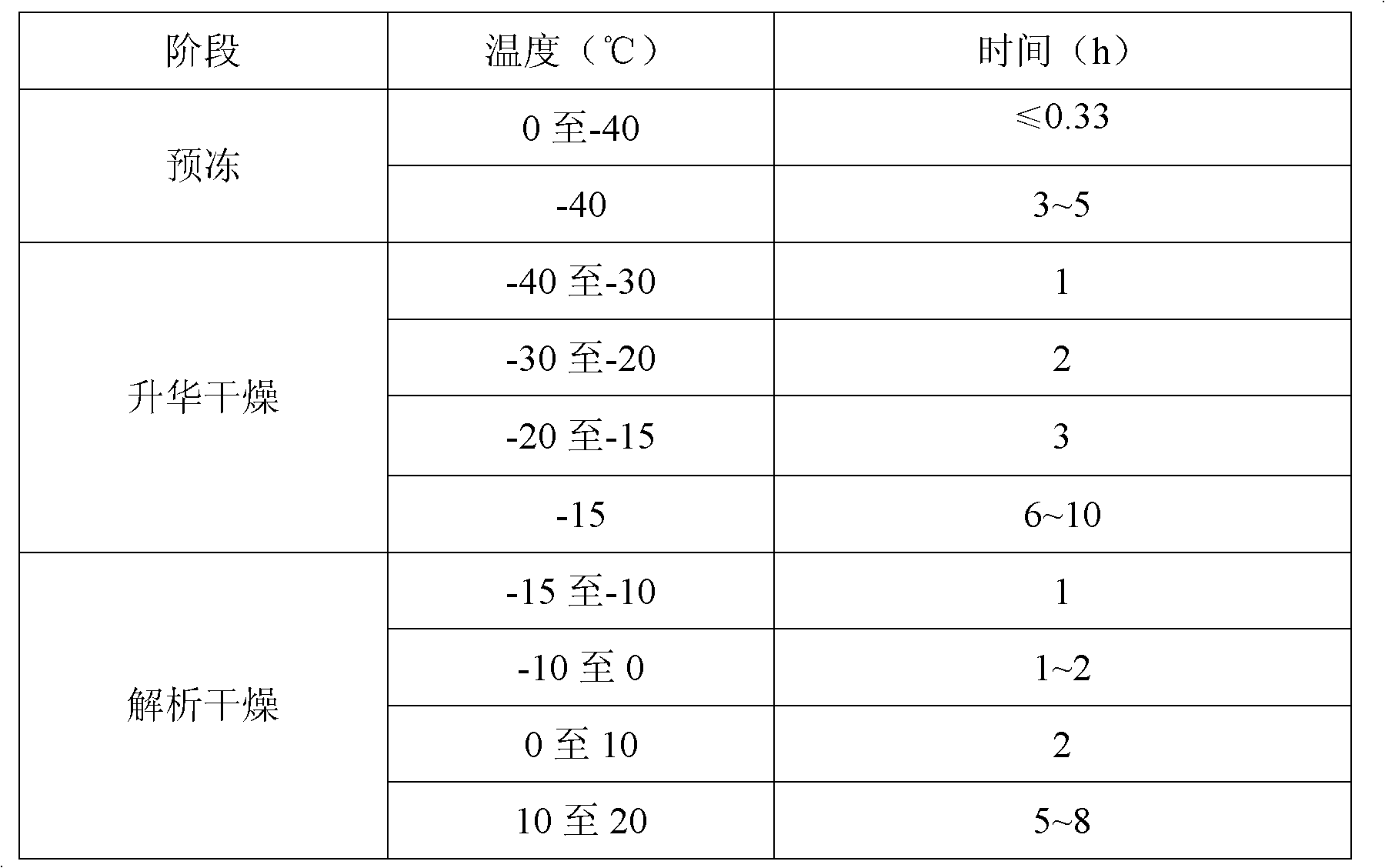Pegylated recombinant human granulocyte colony stimulating factor freeze-dried powder/injection and preparation method thereof
A technology of colony-stimulating factor and PEGylation, which is applied in the field of biomedicine, can solve the problems of molecular structure changes, increase the risk of drug use, and recombined human granulocytes without PEGylation, and achieve the effect of preventing degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Preparation of diluent: all the utensils used have been treated without pyrogens, and the whole process is aseptically operated. Weigh 15g of mannitol, 20g of glycine, 0.492g of glacial acetic acid, and 0.148g of sodium acetate. When the pH of the liquid reaches 4.0, it is sterilized and filtered with a 0.22 μm filter membrane, and the endotoxin content of the diluted liquid measured by the Limulus reagent method should be less than 0.25 EU / mL;
[0025] 2) Preparation of semi-finished products: Take the stock solution of PEGylated recombinant human granulocyte colony-stimulating factor that meets the quality standards, filter it with a 0.22 μm filter membrane to sterilize and filter, measure the protein concentration, and dilute it to PEGylated recombinant human with freshly prepared diluent. The concentration of granulocyte colony-stimulating factor is 1.0mg / mL, and the semi-finished product is obtained;
[0026] 3) Filling and freeze-drying: Fill the semi-finished ...
Embodiment 2
[0032] 1) Preparation of diluent: all the utensils used have been treated without pyrogens, and the whole process is aseptically operated. Weigh 13g of mannitol, 17g of glycine, 0.356g of glacial acetic acid, and 0.162g of sodium acetate. When the pH of the solution reaches 6.3, the 0.22μm filter membrane is sterilized and filtered, and the endotoxin content of the diluted solution measured by the Limulus reagent method should be less than 0.25EU / mL;
[0033] 2) Preparation of semi-finished products: Take the stock solution of PEGylated recombinant human granulocyte colony-stimulating factor that meets the quality standards, filter it with a 0.22 μm filter membrane to sterilize and filter, measure the protein concentration, and dilute it to PEGylated recombinant human with freshly prepared diluent. The concentration of granulocyte colony-stimulating factor is 2.0mg / mL, and the semi-finished product is obtained;
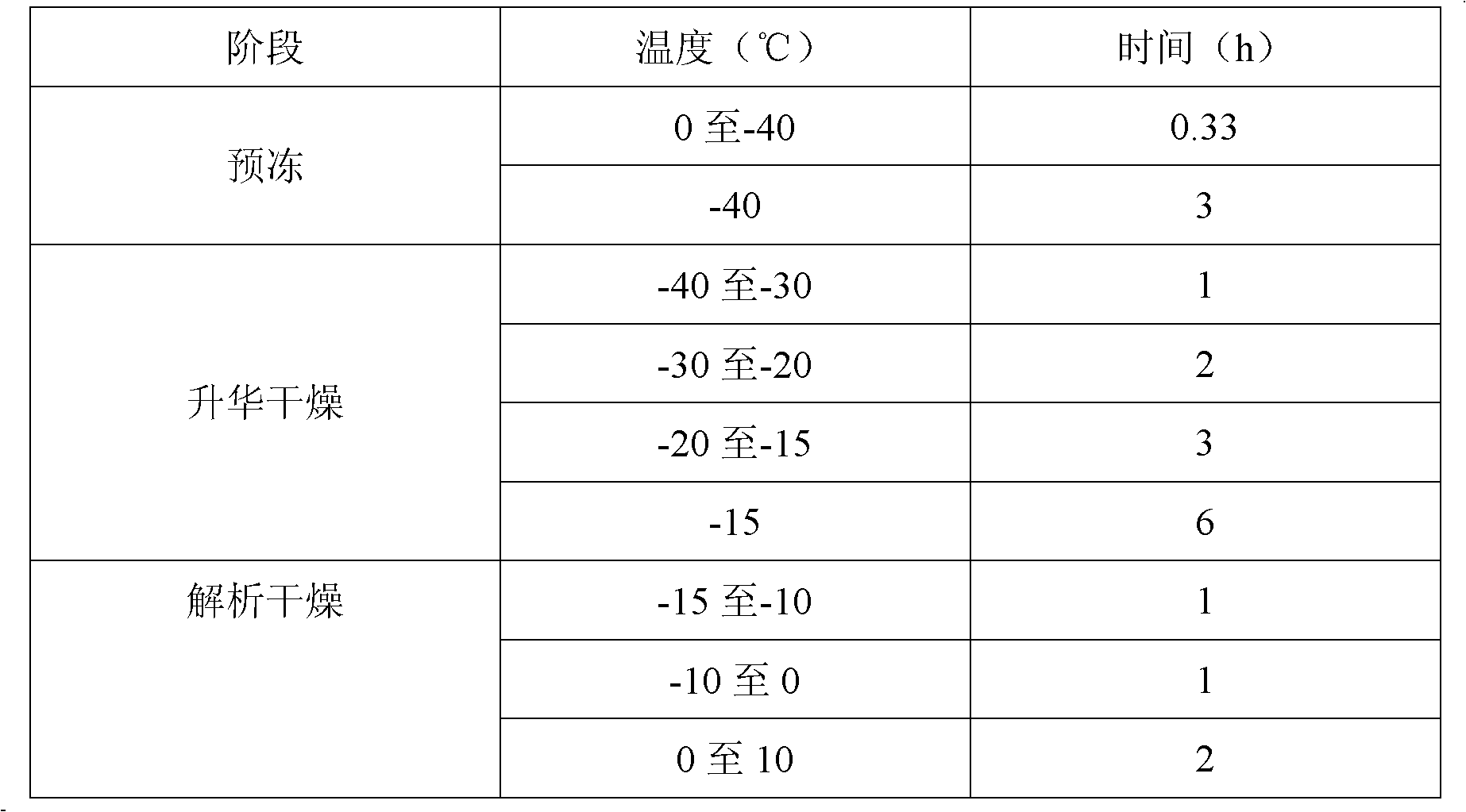
[0034]3) Filling and freeze-drying: Fill the semi-finished produ...
Embodiment 3
[0039] 1) Preparation of diluent: all the utensils used have been treated without pyrogens, and the whole process is aseptically operated. Weigh 15g of glycine, 0.412 glacial acetic acid, and 0.153 sodium acetate, and use the level for injection to determine the volume to 1000mL. Adjust the pH of the diluent to 3.2. 0.22μm filter membrane to sterilize and filter, and the endotoxin content of the diluent measured by the Limulus reagent method should be less than 0.25EU / mL;
[0040] 2) Preparation of semi-finished products: Take the stock solution of PEGylated recombinant human granulocyte colony-stimulating factor that meets the quality standards, filter it with a 0.22 μm filter membrane to sterilize and filter, measure the protein concentration, and dilute it to PEGylated recombinant human with freshly prepared diluent. The concentration of granulocyte colony-stimulating factor is 3.0mg / mL, and the semi-finished product is obtained;
[0041] 3) Filling and freeze-drying: Fill ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com