Preparation method of PAP/GM-CSF (prostatic acid phosphatase/grain macrophage-colony stimulating factor)
A technology of colony stimulating factor and acid phosphatase, applied in the field of preparation of recombinant fusion protein, can solve the problems of weak immunogenicity of PAP and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] The invention provides a method for preparing a preferred PAP / GM-CSF fusion protein, which specifically includes the steps of:
[0111] (1) Cloning the gene encoding the PAP / GM-CSF fusion protein:
[0112]By chemical synthesis, a series of overlapping primers were synthesized, and HindⅢ and XhoI restriction sites were introduced into the upstream primer and downstream primer respectively. After PCR amplification, the optimized DNA sequence of PAP / GM-CSF can be obtained. coding sequence.
[0113] (2) Construct the recombinant expression vector expressing PAP / GM-CSF fusion protein:
[0114] Digest, purify, connect and transform the PAP / GM-CSF sequence and the expression vector sequence; screen and identify positive recombinant plasmids containing the target gene.
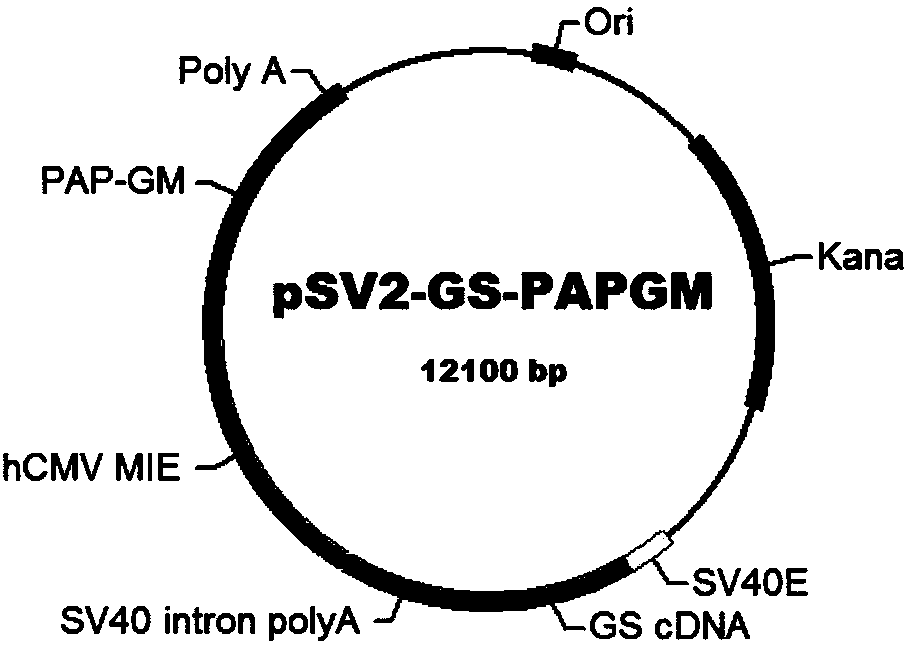
[0115] Many expression vectors can be used for eukaryotic expression of PAP / GM-CSF, including the pSV2 series of vectors. The present invention preferably uses pSV2-GS as the expression vector. The pSV2-GS ...
Embodiment 1
[0135] Example 1 Construction and screening of high expression cell line CHO / pSV2-GS-PAPGM
[0136] The present invention improves the DNA coding sequence of natural human PAP / GM-CSF (as shown in SEQ ID NO.: 3), optimizes it according to codon bias without changing its amino acid sequence, and obtains DNA coding sequence as shown in SEQ ID NO.:4. However, the inventors found that obtaining an optimized sequence based solely on codon preference is not suitable for expression in CHO cells.
[0137] Therefore, the inventors have also carried out targeted secondary optimization based on other factors, including eliminating secondary structures (such as hairpin structures) that are not conducive to expression, changing GC content, CpG dinucleotide content, and the secondary structure of mRNA. Structure, cryptic splice sites, early polyadenylation sites, internal ribosome entry and binding sites, negative CpG islands, regions of RNA instability, repetitive sequences (direct repeats...
Embodiment 2
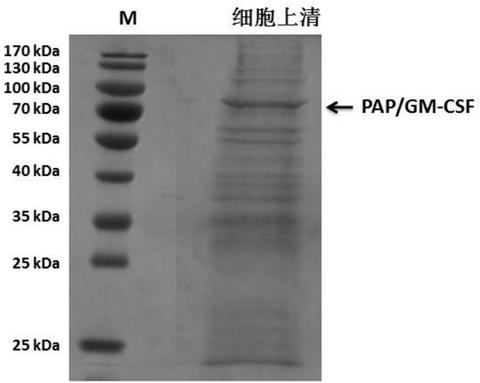
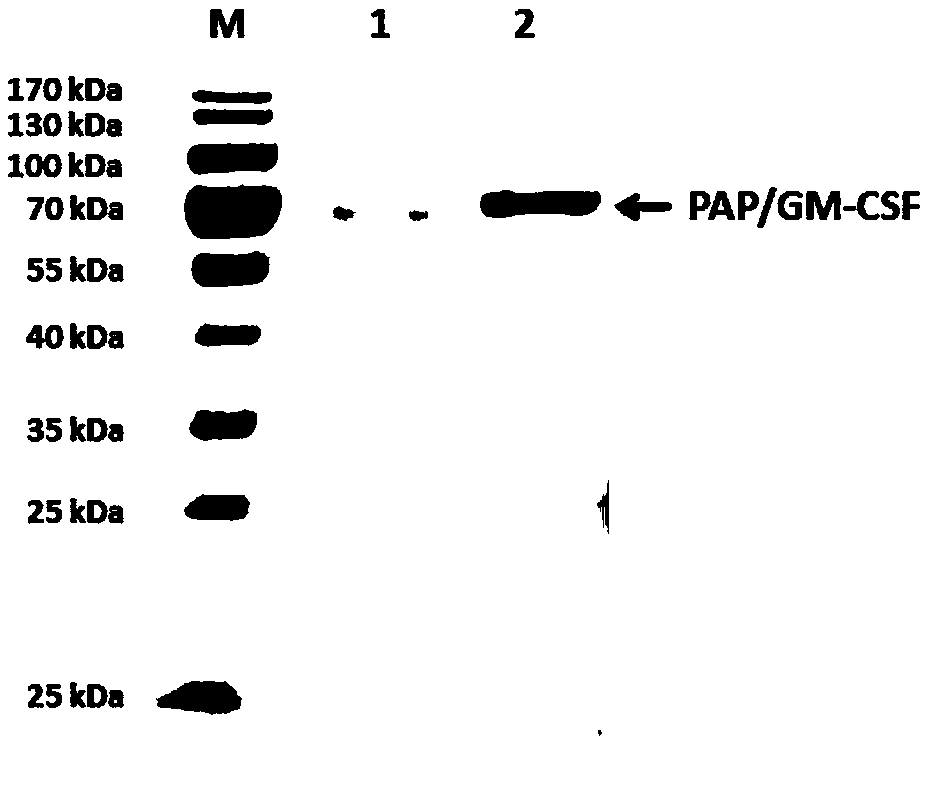
[0180] Example 2 Large-scale cultivation of high expression cell line CHO / pSV2-GS-PAPGM
[0181] Studies have shown that CHO cell lines in CD OptiCHO TM Rapid growth in culture medium, high expression level, and considering CDOptiCHO TM The medium is a chemically defined, protein-free formula that contains no hydrolysates and no components of animal origin. So choose CD OptiCHO TM as a culture medium.
[0182] Resuscitate the CHO / pSV2-GS-PAPGM cell line seed cell in the embodiment 1 from the cell bank, with 2 * 10 5 ~3×10 5 Cells / mL live cell density seeding, placed at 37 ° C, 5% CO 2 Cultured in a humidified incubator as seed cells for experiments.
[0183] Take GS-CHO cells in the logarithmic growth phase, centrifuge at 800r / min for 5min, discard the supernatant, and suspend the cells with fresh basal medium. Take about 3 x 10 5 The viable cell density of cells / mL was inoculated into the reactor, and the culture volume was 1.5L. Reactor operating conditions: pH (7.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com