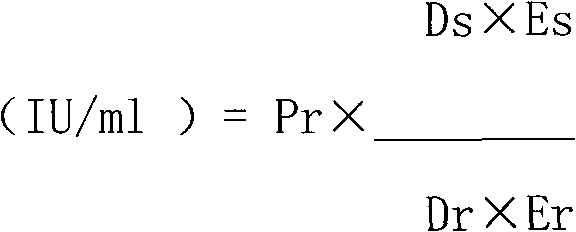Medical preparation containing recombinant human granulocytecolony stimulating factor
A technology of colony stimulating factor and pharmaceutical preparation, applied in the field of biopharmaceuticals, can solve problems such as difficulty in excision of methionine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1. Cloning of human granulocyte colony-stimulating factor without methionine at the N-terminus
[0019] The expression vector pBV220 was donated by the State Key Laboratory of Viral Genetic Engineering, Institute of Virology, Chinese Academy of Preventive Medicine. The recipient strain DH5α is a strain preserved by our company, and its genotype is SupE44△lac U169(80lacZ△M15)hsdR17recALend Al gyr A96thi-1rel AL. Plasmid DNA extraction and purification: refer to the alkaline denaturation method in "Molecular Cloning" edited by Sambeook J et al. (Chinese version, second edition, translated by Jin Dongyan et al., p16).
[0020] Primer design: through computer-aided PCR primer design, the two primers have no hairpin structure, no complementarity of more than 4 bases, and the upstream primer (G+C) is 47.6%, which is an ideal primer design.
[0021] The upstream primers were: - AAGCTT ATG CCA TTA GGC CCT-3'. The first code was originally CCC, but because of the low f...
Embodiment 2
[0028] Embodiment 2. fermentation purification
[0029] Seed medium formula: 1000mL contains 5g of yeast powder, 10g of peptone, 10g of sodium chloride, 2g of glucose, adjust the pH to 7.2-7.4 with sodium hydroxide, and autoclave at 121°C for 20 minutes. Fermentation medium formula: 5g of peptone per liter, Na 2 HPO 4 2H 2 O 6g, KH 2 PO 4 3g, NH 4 CL 1g, NaCl 0.5g, MgSO 4 ·7H 2 O 0.13g, glucose 4g (the latter two components need to be autoclaved separately). The above medium was autoclaved at 115°C for 30 min.
[0030] Seed culture:
[0031] The primary seeds were shaken and cultivated at 30°C and 140r / min for 10h, then transferred to the second-grade seeds with 0.5% inoculum, 30°C and 140r / min were shaken overnight (15h), and transferred to the fermenter with 5% amount.
[0032] Fermenter culture:
[0033] Incubate at 30°C for 3-4h, OD 600 When >1.5, increase the culture temperature to 42°C, continuously aerate for 3.5 hours, and finally collect the bacteria by c...
Embodiment 3
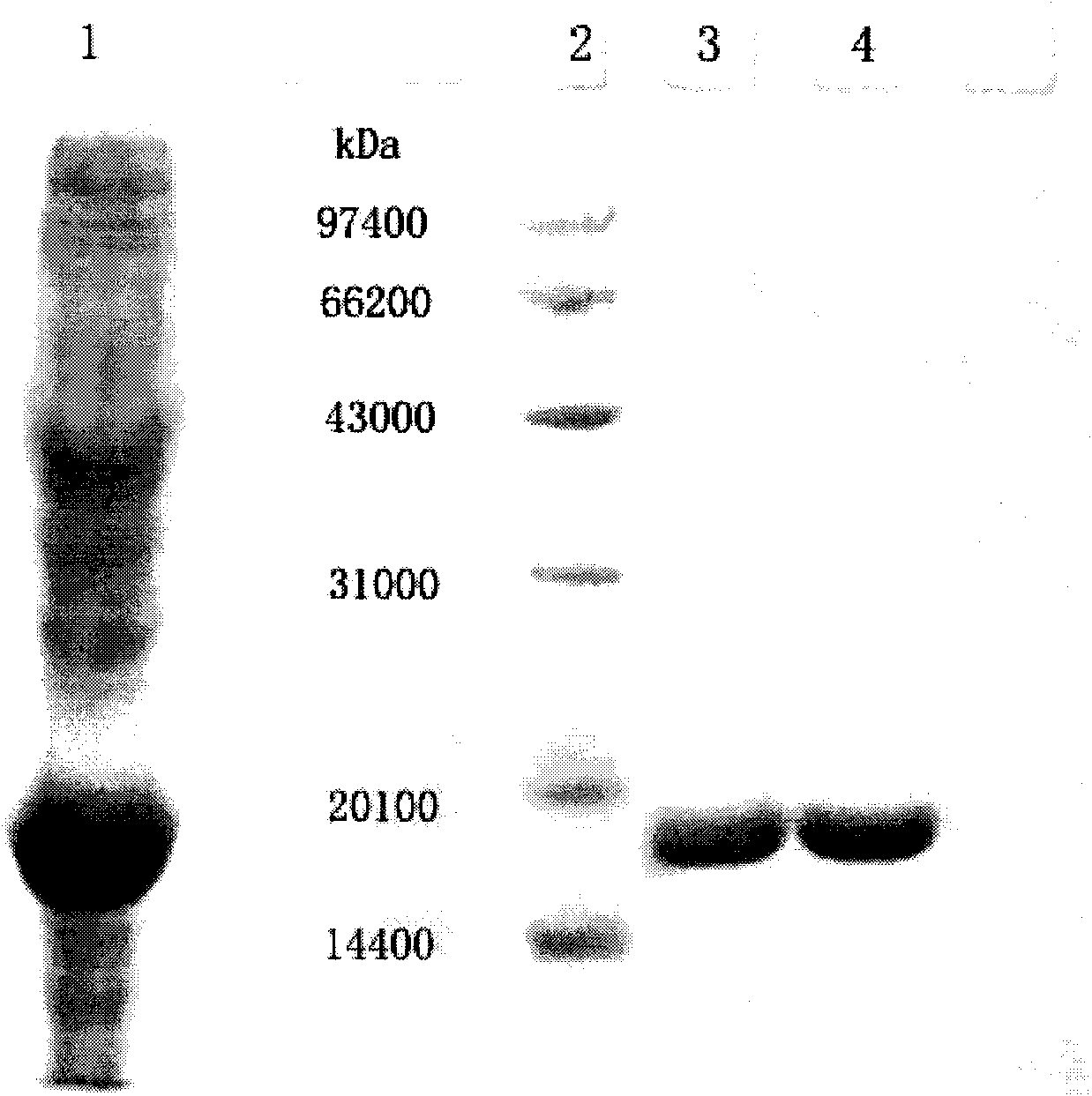
[0056] Example 3 Activity Determination of Recombinant Human Granulocyte Colony Stimulating Factor
[0057] The biological activity of G-CSF was determined by MTT colorimetry. The specific activity that embodiment 1 sample records is 8 * 10 7 IU / mg. The specific method is as follows:
[0058] Reagent: RPMI1640 culture solution: Add 2.0g sodium bicarbonate, 2.383g HEPES, 0.2923L-glutamine per liter, filter and sterilize through a 0.22μm filter membrane. Store at 4°C; basal culture medium: take 100ml of fetal bovine serum (FBS), add it to 900ml RPMI1640 culture medium, store at 4°C; complete culture medium: add recombinant human granulocyte colony-stimulating factor to the basal culture medium to a final concentration of 20ng / ml or 3000IU / ml; Phosphate buffered saline (PBS): Weigh 8g sodium chloride, 0.2g potassium chloride, 1.44g disodium hydrogen phosphate, 0.24g potassium dihydrogen phosphate, add water to dissolve into 1000ml, after 121℃, 15 Sterilize by autoclaving in m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com