Preparation with double functions of stopping bleeding and sustainedly releasing granulocyte colony stimulating factor and preparation method thereof
A colony-stimulating factor, dual-action technology, which can be used in medical preparations containing active ingredients, extracellular fluid diseases, blood diseases, etc. Incomplete and other problems, to achieve the effects of rapid degradation, convenient administration, and good linearity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation and method of preparation with dual effects of hemostasis and sustained release of granulocyte colony-stimulating factor (G-CSF)
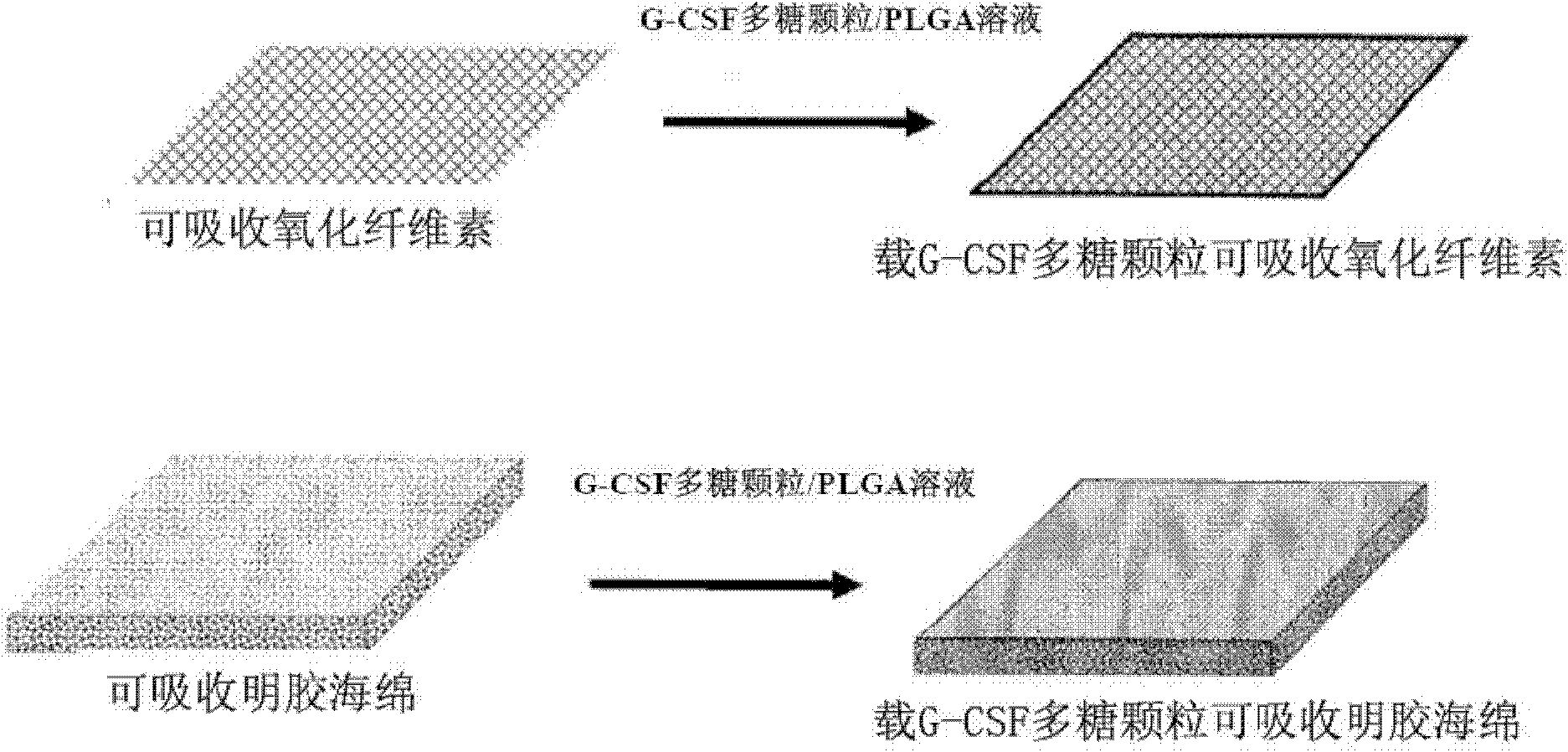
[0042] ①Take a piece of absorbable oxidized cellulose Surgicel (10cm*1cm) and unfold it;
[0043] ②Weigh 0.25mg of polylactic-glycolic acid (PLGA, molecular weight 47kD, lactic acid:glycolic acid=50:50), dissolve in 1.25mg of ethyl acetate for later use;
[0044] ③ Weigh 0.02 mg of dextran particles containing 0.01 mg of granulocyte colony-stimulating factor (the particle size of dextran particles is 5 μm ± 4 μm);
[0045] ④Put the granulocyte colony-stimulating factor dextran granules of ③ into the solution of ②, mix thoroughly and evenly, and obtain a suspension;
[0046] ⑤ Spread the suspension of ④ on the absorbable oxidized cellulose Surgicel with a small brush;
[0047] ⑥Dry ⑤ to get the finished product.
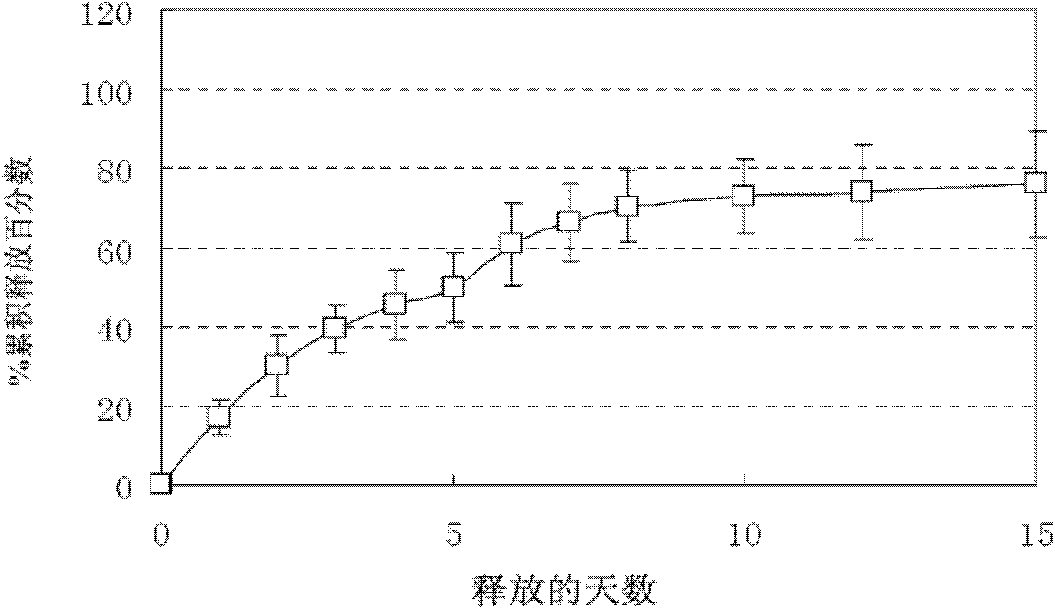
[0048] ⑦In vitro release curve see image 3 , showing good release kinetics.
[0049] ⑧Using the finis...
Embodiment 2
[0053] Example 2: Preparation and method of preparation with dual effects of hemostasis and sustained release of granulocyte colony-stimulating factor (G-CSF)
[0054] ①Take a piece of absorbable oxidized cellulose Surgicel (2cm*1cm) and unfold it;
[0055] ②Weigh 60 mg of polylactic-glycolic acid (PLGA, molecular weight 47kD, lactic acid: glycolic acid=65:35), dissolve in 300 mg of ethyl acetate for later use;
[0056] ③ Weigh 6 mg of dextran particles containing 1.5 mg of granulocyte colony-stimulating factor (the particle size of dextran particles is 5 μm ± 4 μm);
[0057] ④Put the granulocyte colony-stimulating factor dextran granules of ③ into the solution of ②, mix thoroughly and evenly, and obtain a suspension;
[0058] ⑤ Spread the suspension of ④ on the absorbable oxidized cellulose Surgicel with a small brush;
[0059] ⑥Dry ⑤ to get the finished product.
[0060] ⑦In vitro release curve see Figure 4 , showing good release kinetics.
[0061] ⑧Using the finished ...
Embodiment 3
[0064] Example 3: Preparation and method of preparation with dual effects of hemostasis and sustained release of granulocyte colony-stimulating factor (G-CSF)
[0065] ①Take a piece of absorbable oxidized cellulose Surgicel (2cm*1cm) and unfold it;
[0066] ②Weigh 1200mg of polylactic-glycolic acid (PLGA, molecular weight 47kD, lactic acid: glycolic acid=50:50), dissolve in 6000mg of ethyl acetate for later use;
[0067] ③ Weigh 60 mg of dextran particles containing 3 mg of granulocyte colony-stimulating factor (the particle size of dextran particles is 5 μm ± 4 μm);
[0068] ④Put the granulocyte colony-stimulating factor dextran granules of ③ into the solution of ②, mix thoroughly and evenly, and obtain a suspension;
[0069] ⑤ Spread the suspension of ④ on the absorbable oxidized cellulose Surgicel with a small brush;
[0070] ⑥Dry ⑤ to get the finished product.
[0071] ⑦In vitro release curve see Figure 5 , showing good release kinetics.
[0072] ⑧Using the finished ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com